Biology Reference
In-Depth Information
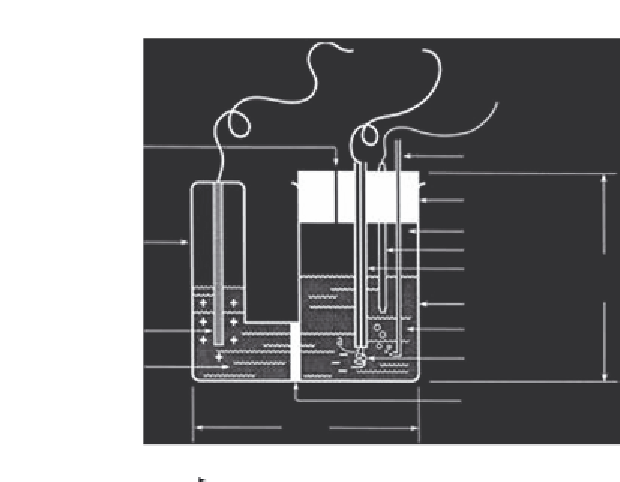
Coulometer
Cell vent
Sample inlet (from SOMMA)
Rubber cap
Headspace
Temperature sensor
Glass insulator
Side-arm
Primary chamber
Cathode solution
Anode electrode(Ag)
Cathode electrode (Pt)
Anode solution
Glass frit
8.5 cm
+
1
2
Ag
O
Ag
+
e
-
e
-
OH
-
+
H
2
O
+
H
2
Anode
Cathode
Figure 6.4
Typical arrangement for a coulometric sensor made of a cathodic and anodic
half reactions for the coulometric titration of the H+ from the acid formed by the reac-
tion of CO
2
and ethanolamine. Source:
Adapted from Ref.
13
.
Furthermore, the calculation of the analyte concentration is straightforward
because the charge is simply the product between the fixed current and the
time. However, it is more difficult to ensure 100% current efficiency and to
know when the electrolysis process is ended and when refinements in the
measurement procedure are required. Controlled-current coulometry is car-
ried out using an amperostat composed of a working electrode, often made of
platinum, and a counter electrode. This counter electrode can be isolated from
the analyte by a salt bridge. The setup also needs an accurate clock for measur-
ing the electrolysis time and a switch for starting and stopping the electrolysis.
The smallest concentration of analyte that can be determined by cou-
lometry depends on the capability to accurately determine the endpoint
of electrolysis. At best, it can nowadays reach the micromolar range. When
using controlled-current coulometry, an accuracy of 0.1%-0.3% is feasible.
In controlled-potential coulometry, an accuracy better than 0.5% is achiev-
able. Precisions of ±0.1%-0.3% are routinely obtained in controlled-current
coulometry, and precisions of ±0.5% are typical for controlled-potential
coulometry.



Search WWH ::

Custom Search