Environmental Engineering Reference
In-Depth Information
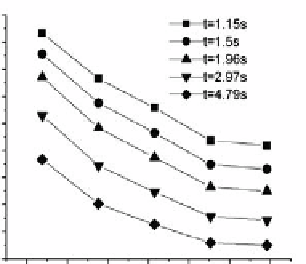
ppm HCl concentrations, 373 or 1,173 K reaction temperatures, 1.15 4.79 s or
3.6315.05 s residence time, respectively. With the increasing concentration of Hg
0
inlet mercury in the same residence time, the concentration of Hg
0
gradually in-
creased, thus leading to reduced transformation rate.
7
6
5
8
t
=3.625 s
t
=4.701 s
t
=6.174 s
t
=9.326 s
t
=15.05 s
t
=1.15 s
t
=1.5 s
t
=1.96 s
t
=2.97 s
t
=4.79 s
7
6
5
4
3
2
1
4
3
2
1
2 4 6 8 10 12
2 4 6 8 10 12
Inlet mercury concentration (g/m
3
)
Inlet mercury concentration (g/m
3
)
Fig. 4.60
Impact of inlet mercury concen-
tration on Hg
0
oxidization, at 80 ppm HCl,
373 K, different residence time from 3.63 to
15.05 s
Fig. 4.59
Impact of inlet mercury concentration
on Hg
0
oxidization, at 20 ppm HCl, 1173 K,
different residence time from 1.15 to 4.79 s
Figs. 4.61 and 4.62 present the impact of Cl/Hg on the transformation of
mercury speciation with the reaction condition of Hg
0
inlet concentration of
6.478 g/m
3
, reaction temperatures of 373 or 1173 K, and HCl concentrations
of 20 or 60 ppm, residence time of 3.63 15.05 s or 1.15 4.79 s, respectively.
As shown in the figures, Cl/Hg increased while the concentration of Hg
0
dropped. It meant that higher Cl/Hg increased the oxidation of Hg
0
under cer-
tain conditions. Cl/Hg could be considered a parameter in the discussion of
regulating mercury oxidation in coal-fired flue as.
5.0
4.5
4.0
3.5
3.0
2.5
6.0
t
=1.15 s
t
=1.5 s
t
=1.96 s
t
=2.97 s
t
=4.79 s
5.0
4.0
t
=3.625 s
t
=4.701 s
t
=6.174 s
t
=9.326 s
t
=15.05 s
2.0
1.5
3.0
1.0
0.5
20000
40000
60000 80000 100000
120000
140000
20000
40000
60000 80000 100000
120000
140000
Cl/Hg
Cl/Hg
Fig. 4.61
Impact of Cl/Hg on Hg
0
oxidiza-
tion at 60 ppm HCl, 373 K, different resi-
dence time from 3.63 to 15.05 s
Fig. 4.62
Impact of Cl/Hg on Hg
0
oxidiza-
tion, at 20 ppm HCl, 1173 K, different resi-
dence time from 1.15 to 4.79 s















Search WWH ::

Custom Search