Environmental Engineering Reference
In-Depth Information
Transformation in Flue Gas
temperature was close to 1,000 K, the final product was 2% Hg
0
, 20% HgCl, and
78% HgCl
2
[15]
. These values were higher than the predicted results in this section.
The difference could be attributed to the parameter options in the calculation and
the differences in experimental and calculation conditions in the current work.
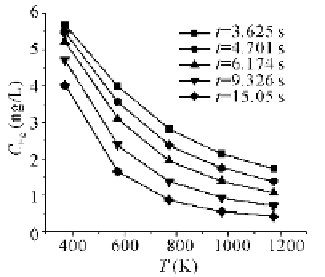
Fig. 4.56
Impact of temperature on Hg
0
oxi-
dization at 40 ppm HCl, residence time from
3.63 to 15.05 s
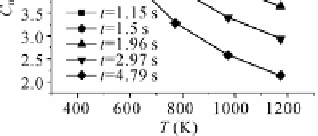
Fig. 4.55
Impact of temperature on Hg
0
oxidization at 20 ppm HCl, residence time
from 1.15 to 4.79 s
Fig. 4.57 and Fig. 4.58 present the impact of residence time on the transforma-
tion of mercury speciation with the reaction conditions of 6.478 g/m
3
Hg
0
inlet
concentration, 60 or 20 ppm HCl concentration, and 3731173 K reaction, respec-
tively. When all other conditions were unchanged, the increase in gas residence time
decreased the amount of Hg
0
. It meant that a longer retention period increased the
oxidation of Hg
0
, and that mercury oxidation in flue gas usually was far from a
theoretically balanced state because residence time was usually less than reaction
time in theory.
Fig. 4.58
Impact of residence time Hg
0
oxi-
dization at 20 ppm HCl, temperatures from
373 to 1173 K
Fig. 4.57
Impact of residence time Hg
0
oxidization at 60 ppm HCl, temperatures
from 373 to 1173 K
Fig. 4.59 and Fig. 4.60 present the impact of Hg
0
inlet mercury concentration on
the transformation of mercury speciation with the reaction conditions of 20 or 80


Search WWH ::

Custom Search