Environmental Engineering Reference
In-Depth Information
60
Original Hg
0
-laden
H
2
O leaching
H
2
SO
4
leaching
FeCl
3
leaching
Na
2
CO
3
leaching
NaOH leaching
Standard TCLP
50
40
30
20
10
0
BLC
FeCl
3
-AC
MnO
2
-AC
ACs
Fig. 5.38
The results of Hg stability on the three Acs
Many forms of mercury that existed on the surface of AC(MnO
2
-MZ), such as
Hg(NO
3
)
2
, Hg
2
(NO
3
)
2
, and some Hg
0
, were attributed to the influence of MnO
4.
Mn
4+
, NO
3
-
, or others. Furthermore, both forms of mercury were easily soluble in
water and in an acid solution. For AC(FeCl
3
-MZ), the amount of Hg that leached
out was between that from AC(MZ) and AC(MnO
2
-MZ); Fe
3+
was estimated as the
only probable one out of all the possible oxidizing mediums, and Cl
was the pri-
mary anion on the surface of AC(FeCl
3
-MZ). Therefore, the oxidizability of
AC(FeCl
3
-MZ) may be weak in contrast with that of AC(MnO
2
-MZ). Conse-
quently, both HgCl
2
and Hg
2
Cl
2
were the most possible existing forms of Hg on the
surface of AC(FeCl
3
-MZ). Moreover, Hg
2
Cl
2
could be the main existing matter for
the number of Cl
-
existing on the surface of AC(FeCl
3
-MZ). Consequently, although
both AC(MnO
2
-MZ) and AC(FeCl
3
-MZ) have high mercury sorption capacities as
an effect of the oxidation treatment, mercury is much more stable in AC(FeCl
3
-MZ)
than in AC(MnO
2
-MZ) in a solution environment because Hg
2
Cl
2
is slightly solu-
ble. Hence, it is very important to first consider the stability of mercury on the
surface of sorbents after adsorption in certain conditions if using an oxidation
treatment to enhance the mercury sorption capacity of sorbents, including AC.
5.5.2.3
Mercury Stability in Activated Carbon Sorbents in Thermal Desorp-
tion
For the coal-fired mercury emission control technology of AC injection, the thermal
desorption characteristics of mercury on the AC are very important in avoiding
re-emission and ensuring safe disposal of combustible byproducts. The experiment
was carried out in air with the aid of an aqueous thermostat to maintain the desired
temperature. The experiments were conducted to investigate the influence of
heating time on mercury released from AC at 60 and 90 °C. The quantity of Hg
0
adsorbed originally in AC was 0.41 g/g, and the amount of Hg
2+
adsorbed origi-





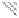



























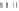










































































Search WWH ::

Custom Search