Environmental Engineering Reference
In-Depth Information
show the leaching results of AC(MZ), AC(MnO
2
-MZ), and AC(FeCl
3
-MZ) using
five different leaching liquids continuously for 14 days and with the standard TCLP.
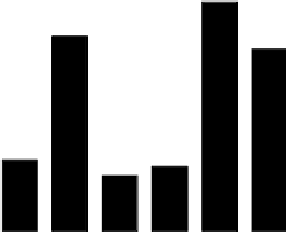
The average Hg concentration in 1% NaOH leachate (pH>14) was about 0.89 μg/L,
which was close to the average Hg concentration of about 0.78 μg/L in 1% H
2
SO
4
leachate (pH<1) (Fig. 5.35). However, this value was much higher than the average
Hg concentrations in H
2
O, 1% FeCl
3
, and 1% Na
2
CO
3
leachates (3<pH<10), which
mostly ranged from 0.22 to 0.28 μg/L. It seemed that Hg was easily released from
the surface of the AC(MZ) in a strong alkaline or acid solution. Compared with the
Hg concentration of the TCLP leachate, which was about 0.73 μg/L, the Hg con-
centrations in all the leachates were still lower than the safe limit defined by the
Universal Treatment Standard, which limits content of mercury in the TCLP
leachate to 25 μg /L. Based on these experiments, Hg was found to be stable on the
surface of the AC(MZ) in the solution.
1.0
BLC Leaching
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0.0
H
2
OH
2
SO
4
FeCl
3
Na
2
CO
3
NaOH
TCLP
Different leaching methods
Fig. 5.35
The leaching results of AC(MZ)
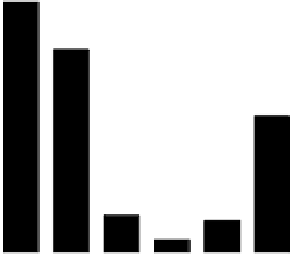
90
MnO
2
-AC Leaching
80
70
60
50
40
30
20
10
0
H
2
OH
2
SO
4
FeCl
3
Na
2
CO
3
NaOH
TCLP
Fig. 5.36
The leaching results of AC(MnO
2
-MZ)
Different leaching methods























































Search WWH ::

Custom Search