Environmental Engineering Reference
In-Depth Information
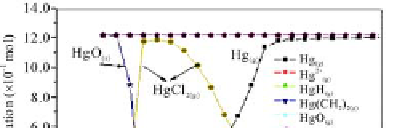
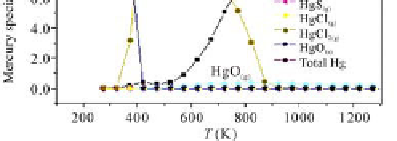
temperature range of 50 - 60 °C, the mercury speciation had HgCl
2(g)
, HgO
(s)
, Hg
(g)
,
HgO
(g)
and HgS
(g)
, which were mainly gaseous HgCl
2
and solid HgO.
Fig. 5.32
The calculation results of mercury speciation in desulfurizing tower
The experimental study of the second emission and mercury stability on desul-
furization gypsum as influenced by temperature, humidity, time and so on, is im-
portant for preventing environmental pollution and promoting the effective use of
gypsum.
5.5.1.2 Mercury Stability on Desulfurization Gypsum in Natural Storage
The stability of mercury on gypsum at an environmental temperature was studied.
Fig. 5.33 shows the result of mercury that escaped from gypsum at different tem-
peratures in natural storage. The mercury-escaped ratio of gypsum was relative to
time of storage at environmental temperature. In the natural environment, more
mercury generally escapes within a longer period of time. Compared with mercury
stability in gypsum at an environmental temperature of 50 °C (Fig. 5.33), the
mercury release curve at an environmental temperature was gentler than that at 50
°C, which meant that the amount of mercury that escaped at low temperature was
relatively small.
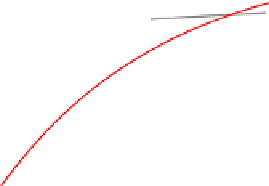
70
40
60
30
50
20
40
Room temperature
y
= 53.76 - 55.16*0.949
x
R
2
=0.9047
50
o
C
y
= 20.438*ln(
x
+1.258)
R
2
=0.69235
10
30
0
20
0
2
4
6
8
10
12
0
5
10
15
20
25
30
Heat-up time (day)
Heat-up time (day)
(a)
(b)
Fig. 5.33
Mercury escaped from gypsum at different temperatures in natural storage







































































































Search WWH ::

Custom Search