Environmental Engineering Reference
In-Depth Information
When Step 2 was finished, the simulated flue gas components were introduced
into the gas flow one by one. Meanwhile, the total flow was kept constant by ad-
justing the N
2
balance gas. The effect of different flue gas components on Hg
0
adsorption was evaluated in this step.
Once all the gas components were added, the adsorption of Hg
0
by AC in the
complete simulated flue gas started. The adsorption time in the Step 4 was quite
long at approximately 41 h and 22 min. The effect of flue gas components on Hg
0
adsorption and the mechanism of Hg
0
adsorption by AC were studied.
Fig. 5.28 shows the outlet Hg concentration on the fixed adsorption bed. Ini-
tially, the rate of Hg
0
adsorption by AC was very high. As the experiment pro-
ceeded, Hg
0
was detected 4 h later, and its concentration reached 14.2 g/(N·m
3
) at
the 16
th
hour; Hg
2+
never emerged during the entire process. The above-mentioned
Hg
0
adsorption phenomenon seemed to indicate that the Hg
0
adsorption by AC in
N
2
may be a physical adsorption. When the Hg
0
adsorption by AC was a physical
adsorption, the saturated adsorption of the AC was reached at the 16
th
hour (Fig.
5.28). Then, more Hg
0
would no longer be adsorbed by AC because all the pores in
the surface of AC were filled by Hg
0
when the AC had reached the saturated ad-
sorption. However, the following adsorption in the simulated coal-fired flue gas
provided interesting and important results.
When the gas flow into the reactor was switched from N
2
to simulated gas
components one by one, the concentration of Hg
0
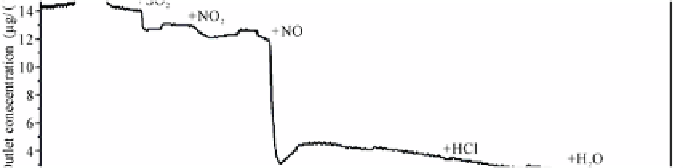
dropped (Fig. 5.28) (Step 3). The
influence of different gas components on Hg
0
adsorption was observed during
switching from N
2
gas to the simulated flue gas from 15:59:50 to 19:29:50 (Fig.
5.29). Excluding CO
2
, almost all other gas components, especially NO, enhanced
Hg
0
adsorption by AC.
Fig. 5.29
Concentrations of Hg
0
and Hg
2+
during switching from N
2
gas to simulated flue gas
Competitive adsorption also existed between CO
2
and Hg
0
on AC. Accordingly,
Hg
0
concentration increased because surface conditions of AC favored CO
2
ad-
sorption when CO
2
was added at 15:59:50.


Search WWH ::

Custom Search