Environmental Engineering Reference
In-Depth Information
adsorption efficiency curves of Hg
0
by lime on the two kinds of simulated flue gas
are shown in Fig. 5.4. The existence of SO
2
was favorable for lime adsorbing Hg
0
.
Based on experiments of Hg
0
adsorption by hydrated lime and lime, chemical
adsorption was found to be the key in the process of Hg
0
adsorption by cal-
cium-based sorbents. SO
2
, improved Hg
0
adsorption by calcium-based sorbents
through the chemical adsorption mechanism.
0.6
no SO
800 ppm SO
100
0.5
90
0.4
0.3
80
0.2
BL
800 ppm SO
2
70
0.1
0.0
60
0
5
10
15
20
25
30
35
0
5
10
15
20
25
30
35
Time (min)
Time (min)
(a) (b)
Fig. 5.4
Hg
0
adsorption by lime on two kinds of simulated flue gas
Hg
0
adsorption ability of MFC
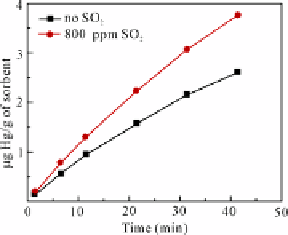
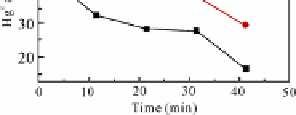
MFC is a mixture of fly ash LFA1 and lime with a mass ratio of 3:1. Adsorption
experiments were conducted on two kinds of simulated flue gas: 1) BL and 2) BL
added with 800 ppm SO
2
. Other conditions were the same (Hg
0
concentration: 21.5
g/m
3
; temperature: 125 °C; flow: 1 L/min). The adsorption kinetic and adsorption
efficiency curves of Hg
0
by lime on the two kinds of simulated flue gas are shown in
Fig. 5.5. The existence of SO
2
was favorable for MFC adsorbing Hg
0
.
The three kinds of calcium-based sorbents, namely hydrated lime, lime and
MFC, had higher adsorption capacities and absorption efficiencies in flue gas with
SO
2
. Calcium-based sorbents are widely used as flue gas desulfurizers, indicating a
certain ability for Hg
0
removal. A combined removal technology of SO
2
and Hg
0
through injection of calcium-based sorbents can be considered.
(a) (b)
Fig. 5.5
Hg
0
adsorption by MFC on two kinds of simulated flue gas




































































































Search WWH ::

Custom Search