Biomedical Engineering Reference
In-Depth Information
to be the excrements of fossil reptiles, also abound in phosphate of
lime.” (p. 332) means that already in 1863 researchers were aware
on this fact.
Furthermore, let me make a citation from a publication by Wells
of 1906 [69]: “The apparent constancy of the proportion of carbonate
and phosphate of calcium in bones made an impression on Hoppe-
Seyler in I862, and we find him speculating on the possibility of
the components of the two salts being joined together to form one
giant molecule: 3 (Ca
, which he imagined might be
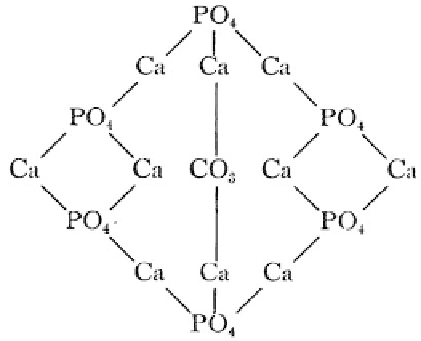
united in some such way” (p. 522) as shown in Fig. 8.1. Further, Wells
mentions: “This formula is interesting chiefly from the historical
standpoint, but it serves to emphasize the tendency of these salts
to appear in nearly constant proportions in the animal body, a
fact possibly of some importance.” (p. 523). Obviously, the atomic
arrangement shown in Fig. 8.1 represents the earliest structural
drawing of a single molecule of carbonateapatite. An attentive reader
will notice two different types of calcium (currently known as Ca(1)
and Ca(2)) in that structure.
(Po
)
)-CaCo
3
4
2
3
Figure 8.1
The first available structure of a bone mineral (carbonatea-
patite). Reprinted from Ref. [69].
Vitrification properties of some forms of calcium orthophosphates
at heating have been known since, at least, 1877 [70]. The modern
chemical formula of perfectly transparent crystals of natural FA as
Ca
F has been known since, at least, 1873 [71], while the major
crystallographic faces of a natural calcium apatite were described in
(PO
)
5
4
3


Search WWH ::

Custom Search