Biomedical Engineering Reference
In-Depth Information
pH, temperature, crystal dimensions, and the presence of chemical
modifiers on the dissolution kinetics. A rotary disk [10, 13, 14],
constant composition [9, 16, 18, 19, 20, 32-34] or dual constant
composition [22, 34, 35] techniques are used for experimental
investigations. The results obtained are usually plotted as an uptake
of H
+
ions (e.g., as a titrant volume added) and/or a release of calcium,
orthophosphate, and fluoride ions versus the dissolution time.
Afterwards, calculations of the numeric values for activation energy,
rate constants, effective reaction order, diffusion layer thickness,
characteristic adsorption impedance, diffusive jump distance,
etc., are performed [9-29, 32-36]. The conclusion on whether a
dissolution process is kinetically or diffusion controlled is made
based on the numeric values calculated. For example, when analysis
of the rate data gave a numeric value of the effective reaction order
n
= 6, a surface controlled dissolution mechanism was suggested
[22].
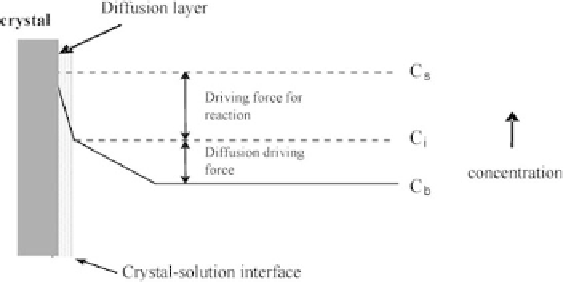
Figure 7.1
A dissolution process according to the diffusion and kinetically
controlled models. Here:
C
— solute concentration on the
s
surface,
—
solute concentration in bulk. Reprinted from Ref. [30] with
permission.
C
— solute concentration on the interface,
C
i
b
Basic thermodynamic principles predict that dissolution rates
should increase with increasing driving force or chemical potential;
however, the experimental studies show that this dependence is
complex. Namely, dissolution of apatite in some cases was found to be
diffusion controlled [19, 20, 23, 25], in some other cases—kinetically
controlled [21, 22] and even intermediate (i.e. both kinetically and
diffusion controlled) in still other cases [14]. Furthermore, the


Search WWH ::

Custom Search