Biomedical Engineering Reference
In-Depth Information
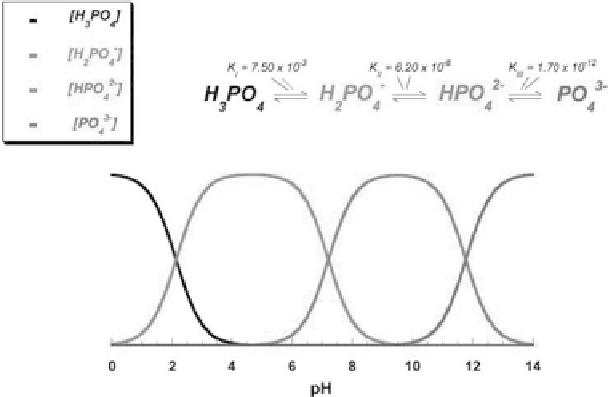
Due to the triprotic equilibrium that exists within
orthophosphate-containing solutions, variations in pH alter the
relative concentrations of the four polymorphs of orthophosphoric
acid (Fig. 1.4) [118] and thus both the chemical composition (Fig.
1.5) [119] and the amount of the calcium orthophosphates that
are formed by a direct precipitation. The solubility isotherms of
different calcium orthophosphates are shown in Fig. 1.6 [28, 29,
110, 111, 120-123]. However, recently, the classic solubility data
of calcium orthophosphates [28, 29, 110, 111, 120-123] were
mentioned to be inappropriate [124]. According to the authors of
the latter study, all previous solubility calculations were based on
simplifications, which are only crudely approximate. The problem
lies in incongruent dissolution, leading to phase transformations
and lack of the detailed solution equilibria. Using an absolute solid-
titration approach, the true solubility isotherm of HA was found to lie
substantially lower than previously reported. In addition, contrary to
a wide belief, DCPD appeared not to be the most stable phase below
pH ~4.2, where CDHA is less soluble [124].
Figure 1.4
pH variation of ionic concentrations in triprotic equilibrium
for phosphoric acid solutions. Reprinted from Ref. [118] with
permission.
A brief description of all known calcium orthophosphates is
given in Table 1.1.


Search WWH ::

Custom Search