Biomedical Engineering Reference
In-Depth Information
2+
coordinated to surface Ca
ions, approximately in the 1 : 1 ratio,
while the OH groups account only for ~20% of the surface hydration
species. The FTIR data indicated that water molecules, located on
the surface of nanodimensional apatites, are coordinated to surface
cations and experience hydrogen bonding significantly stronger than
that in liquid water [208]. The surface hydrated layer is very delicate
and becomes progressively transformed into a more stable apatitic
lattice upon ageing in aqueous media. Furthermore, it irreversibly
altered upon drying [202]. Outgassing at increasing temperatures up
to ~300°C resulted in a complete surface dehydration, accompanied
by a decrease of the capability to re-adsorb water. Combination of
these data with rehydration tests suggested that a significant part of
the surface Ca
ions, once dehydrated, could undergo a relaxation
inward the surface, more irreversibly as the outgassing temperature
increased [207].
2+
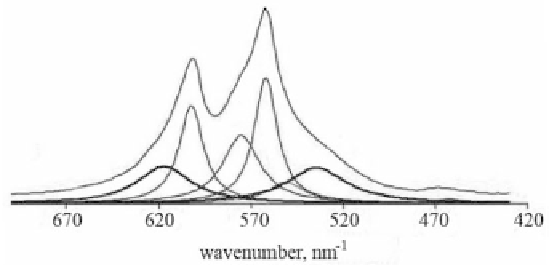
Figure 3.1
FTIR spectra of poorly crystalline apatites showing the non-
apatitic environments of the orthophosphate ions (bold lines
with peaks at 617 and 534 cm
−1
) and the apatitic PO
4
3−
(thin
lines with peaks at 600, 575 and 560 cm
−1
) and HPO
4
2−
(thin
line with peak at 550 cm
−1
) in the
ν
PO
domain. Reprinted
4
4
from Ref. [202] with permission.
In another study, elongated nano-sized crystals of CDHA of ~10
nm thick and of ~30-50 nm length were synthesized followed by
investigations with X-ray diffraction and nuclear magnetic resonance
techniques. The nano-sized crystals of CDHA were shown to consist
of a crystalline core with the composition close to the stoichiometric
HA and a disordered (amorphous) surface layer of 1-2 nm thick [206,
207] with the composition close to DCPD [205]. Based on the total


Search WWH ::

Custom Search