Biomedical Engineering Reference
In-Depth Information
4
2−
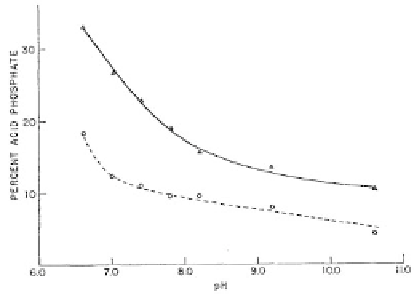
Figure 2.8
Acid phosphate content (percent of total P as HPO
) as
a function of pH for washed (dashed line) and unwashed
(solid line) ACP precipitates. Reprinted from Ref. [137] with
permission.
4
2−
ions in ACP has been confirmed by solid-
state nuclear magnetic resonance (NMR) technique: the results
revealed the presence of ~20% of HPO
The presence of HPO
4
2−
in two ACP samples
[209]. Furthermore, orthophosphate units close to water and a third
(remained unknown) type of orthophosphate groups were found in
the NMR spectra of the ACPs. What's more, substantial differences
were noticed between the NMR spectra of two ACP samples, donated
by two different research groups [209]. In other studies, two ACP
samples of different chemical composition (since they had been
prepared at solution pH = 6.5 and 10.0, respectively) were found to
give very similar EXAFS spectra [133, 210]. The latter means that a
SRO around calcium ions of both ACPs appeared to be very similar;
however, the reasons why the pH differences were not reflected in
the calcium environment remained unclear. These experiments
confirm the fact that ACPs are not a single chemical compound but
represent a special class of amorphous calcium orthophosphate
salts. Thus, precipitated ACPs cannot be described by a single
chemical formula; a sketch Ca
O (Table 1.1) seems
to be the most reasonable element demonstration of this class of
calcium orthophosphate salts. Presumably, ACPs with the Ca/P ratio
exceeding ~1.6 should also contain some amount hydroxide anions;
however, no information on this point has been found in literature.
However, a presence of CaO is frequently detected in ACP-containing
calcium orthophosphate coatings prepared by plasma spraying.
H
(PO
)
·nH
x
y
4
z
2


Search WWH ::

Custom Search