Biomedical Engineering Reference
In-Depth Information
(a)
(b)
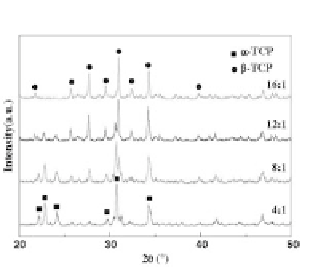
Figure 2.5
X-ray diffraction patterns of: (a) freeze-dried precipitates
prepared from aqueous solutions containing different amounts
of polyethylene glycol. Please, note a shift of the center of the
broadened peak from ~32° toward ~31° with increasing
polyethylene glycol/Ca molar ratio, which implies some
structural differences in the resulting ACPs; (b) heat-treated
(800°C) ACPs prepared from aqueous solutions containing
different amounts of polyethylene glycol. Reprinted from Ref.
[150] with permission.
2.3.2.3
Mechanical and pressure-induced techniques
In addition to the aforementioned solution-based methods, various
types of ACPs might be prepared by dry chemical techniques. For
example, an ACP was prepared using a mechano-chemical method
involving a dry mixture of DCPD and Ca(OH)
reactants with a Ca/P
ratio of 1.67 [155]. Other authors have shown that a prolonged high-
energy ball milling of either α-TCP, β-TCP powder in ethanol or a dry
mixture of ACP and DCPD powders lead to ACP formation after 24 h
[156-158]. Furthermore, prolonged high-energy ball milling of TTCP
was found to result in a mechanical activation with the formation of
undisclosed nanocrystalline and/or amorphous domains within the
compound [159]. However, there is a non-negligible risk of powder
contamination (ball wear) when using this processing route over
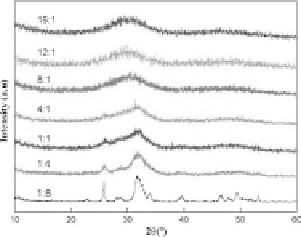
extended periods to obtain an ACP [31]. In addition, a crystalline
to amorphous transition has been detected for various calcium
orthophosphates at very high (up to 10 GPa) pressures (Fig. 2.6)
[160, 161].
2

Search WWH ::

Custom Search