Biology Reference
In-Depth Information
bond of the hydroxyflavanone quercetin
(Fig. 6.19a) to afford a depside. This type of
reaction may proceed through a hydroper-
oxide intermediate, which cyclizes and
decomposes with the loss of carbon monox-
ide or carbon dioxide.
The oxidation of quercetin to the cor-
responding depside also occurs in biologi-
cal systems. However, the precursors of
flavonoids, chalcones, conjugated with car-
bonylic groups favour the Diels-Alder reac-
tion with this species of oxygen (Fig. 6.19b).
A somewhat related reaction is the oxida-
tion of chalcones, which are the biogenetic
precursor of aurones. Sensitized photooxy-
genation of a chalcone proceeds through
dioxetane to yield aurone.
Vitamine E (a-tocopherol and similar
compounds) is also a relatively efficient
singlet oxygen suppressor (Table 6.2) and
is widely used as an antioxidant agent
(Huang
et al
., 2005; Molyneux, 2007;
Nenadis
et al
., 2007). Trolox, which is a water-
soluble derivative of vitamin E, is also used
OH
O
OH
6
OH
OH
HO
O
OH
Myrecetin
OH O
5
OH
Quercetin
HO
O
OH
OH
4
3
CH
3
OH
O
O Rullnoside
HO
Gallic acid
2
O
CH
3
OH
O
HO
H
3
C
O
OH
O
OH
OH
CH
3
OH
O
1
Rutin
Trolox
OH
HO
HO
O
OH
OH
Luteolin
Apigenin
0
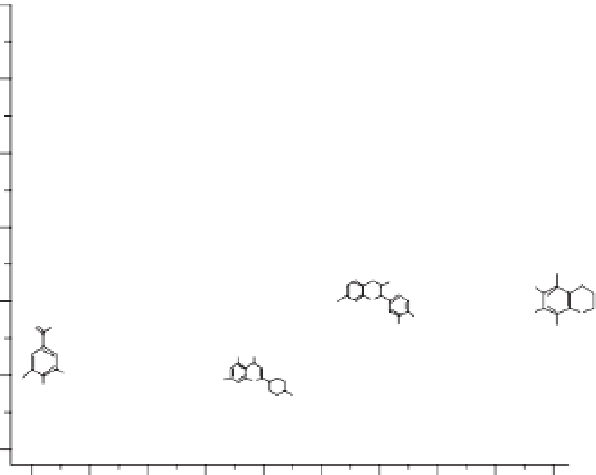
-9.35 -9.30 -9.25 -9.20 -9.15 -9.10
-9.05
-9.00
-8.95 -8.90
HOMO Energy (eV)
Fig. 6.18.
K
Q
versus HOMO energy for a series of flavones, flavonols and similar structures.
OH
OH
OH
HO
O
HO
O
HO
O
(a)
HO
O
OH
OH
OH
1
O
2
O
OH
O
OH
OH O
OH
OH
O
O
Depside
Quercetin
OMe
OMe
MeO
O
O
O-OH
MeO
OH
(b)
MeO
OH
MeO
OMe
OMe
O
1
O
2
O
OMe
O
OMe
O
OMe
O
OMe
O
OMe
MeO
OMe
OMe
Chalcone
Fig. 6.19.
Mechanism of attack of
1
O
2
in the flavonoids (a) quercetin and (b) chalcon.




















Search WWH ::

Custom Search