Biology Reference
In-Depth Information
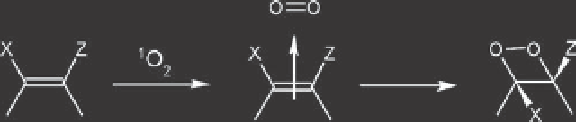
from the same side, suprafacial. However,
an antarafacial route is also possible. The
possible mechanism involves an initial
charge transfer, which is conceivable where
the HOMO of the electron-rich alkene is
higher in energy than the lowest unoccu-
pied molecular orbital (LUMO) of singlet
oxygen. Under these circumstances an
[2s + 2s] addition is allowed suprafacially,
as shown in Fig. 6.7. Orbital symmetry con-
servation concepts are also important in
understanding the thermal decomposition
of 1,2-dioxethane systems, which is allowed
as a concerted process, provided that one of
the carbonyl fragments is formed in the
excited state (Machado
et al
., 1995).
Diels-Alder addition in conjugated
dienes:
The Diels-Alder reaction is a
cycloaddition reaction, resulting from an
electronic reorganization. Two different p
bond-containing molecules react to form a
cyclic compound. Each of the reactants
loses a p bond and the resulting cyclic prod-
uct has two new s bonds. In a cycloaddition
reaction the orbitals of one molecule must
overlap with the orbitals of the other.
Therefore, the frontier molecular orbitals of
both reactants must be evaluated to deter-
mine the outcome of the reaction. Because
the new s bonds in the product are formed
by donation of electron density from one
reactant to the other, we must consider the
HOMO of one of the molecules and the
LUMO of the other. To perform a Diels-
Alder reaction it is necessary that either the
interaction of the LUMO of the dienophile
(2p) and the HOMO of the diene (4p) or the
HOMO of the dienophile and the LUMO of
the diene occur. Singlet oxygen is a good
dienophile, reacting by [4p + 2p] cycloaddi-
tion with the diene resulting in a ring
system, called an endoperoxide (Di Mascio
et al
., 1992, 1997) (Fig. 6.8).
The cyclic peroxides are often rather
unstable and then decompose with explo-
sive results. The reaction by which they
are formed is a concerted symmetry allowed
4p + 2p cycloaddition. Indeed, the dienes
react so rapidly that they can be used as
chemical traps to divert the course of a
reaction under study, suppressing
1
O
2
and/
or providing evidence for an
1
O
2
pathway.
Occasionally, the endoperoxides can be iso-
lated and identified as intermediates. The
cyclopentadiene results in a rather noxious
explosive endoperoxide; however, it can be
stabilized by appropriate phenyl substitu-
tion (Fig. 6.9a,b). Extended aromatic sys-
tems result in an endoperoxide that can be
manipulated at room temperature (Fig. 6.9c).
These reactions are reversible by thermoly-
sis in solution, and singlet oxygen is gener-
ated (Di Mascio
et al
., 1992, 1997).
Furans and isobenzofurans are very
reactive and are sometimes used to quantify
singlet oxygen generation. In both cases, the
endoperoxide is an ozonide; it can be
detected, isolated and characterized at low
temperature (Koch and Schenck, 1966).
1,3-Diphenylisobenzofuran (DPBF) is a flu-
orescent molecule that possesses a highly
specific reactivity towards
1
O
2
, forming an
endoperoxide that decomposes to result in
1,2-dibenzoylbenzene (Fig. 6.10). Measuring
the intensity decrease in absorbance or fluor-
escence of DPBF can follow this reaction
between DPBF and
1
O
2
, which is one of the
most frequent used to determine quantum
yields of
1
O
2
(Spiller
et al
., 1998; Tada
et al
.,
2007; Rossi
et al
., 2008).
By means of these main reactions as well
as other pathways that are described in detail
Charge-transfer
assembly
Fig. 6.7.
Suprafacial attack of singlet oxygen with charge-transfer assembly.
Search WWH ::

Custom Search