Biology Reference
In-Depth Information
formed. Singlet oxygen can also be formed
by chemical/biochemical reactions, espe-
cially by the Russell Mechanism present in
peroxidation processes (Miyamoto
et al
.,
2007), which may indicate a possible role
for singlet oxygen in signalling events
mainly related to the cellular stress response
(Klotz
et al
., 2003).
A small fraction of the population of
singlet oxygen molecules that is formed
decays to the ground state emitting light in
the near infrared region (NIR) l
MAX
= 1268 nm
(Krasnovskii, 1976; Khan and Kasha, 1979;
Wilkinson
et al
., 1993) and this is the spec-
tral fingerprint of singlet oxygen molecules
(Fig. 6.3). This detection method has been
used to observe singlet oxygen
in vivo
in
tissues and
in vitro
in different types of
solutions and/or suspensions (Niedre
et al
.,
2002; Kuimova
et al
., 2009). Usual detection
equipment includes a laser system to
provide light excitation and generation of
singlet oxygen and a NIR fluorometer to
detect its characteristic emission (Fig. 6.3).
Singlet oxygen can also be detected and
quantified using chemical trap methods and
extremely selective probes have been
designed. Natural molecules, such as beta-
nidines found in beetroot, have also been
shown to work well as a probe to detect and
quantify
1
O
2
(Bonacin
et al
., 2009).
Singlet oxygen can be generated in a
controlled and reproducible way either by
chemical or by physical methods. The most
used chemical methods are: the reaction
between hydrogen peroxide and sodium
hypochlorite, N-chlorosuccinimide and
alkaline hydrogen peroxide; and the ther-
molysis of several endoperoxides (Baptista,
1998). By physical methods, it is possible
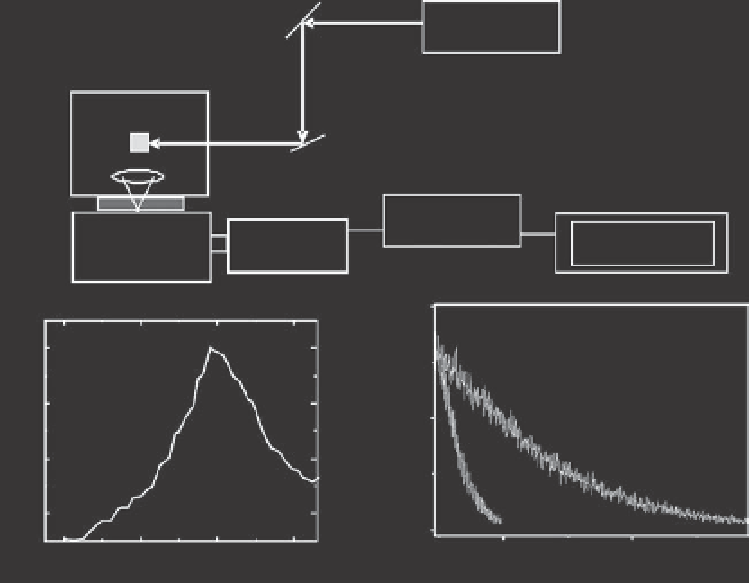
LASER
(a)
SAMPLE
F
CONTROL
COMPUTER
NIR-PMT
MONO
(b)
(c)
12.0
1.0
0.8
6.0
0.6
(i)
(ii)
0.4
0.0
5.0
10.0
1200
1240
1280
1320
Time (
µ
s)
Wavelength (nm)
Fig. 6.3.
(a) Experimental set-up to prove and study the generation and reactivity of singlet oxygen.
Equipment is built with laser sources that are used to excite photosensitizer molecules that form triplet
states and react with oxygen forming
1
O
2
. The light emission is filtered with silicon and/or interference filters
(F), passes through a monochromator and is detected either by a NIR-PMT (faster and more sensitive) or by
a Germaniun detector. (b) Characteristic NIR emission spectra of
1
O
2
generated in aqueous solution of
Methylene Blue (10
m
M) after excitation at 532 nm (10 mJ/pulse, 10 Hz). (c) Transient decay of
1
O
2
in
aqueous solution, the lifetime of which is ~3
m
s (i) and in aqueous solution in the presence of 1 mM sodium
azide (ii) which is a commonly used agent that suppresses
1
O
2
and consequently reduces its lifetime.
Search WWH ::

Custom Search