Biology Reference
In-Depth Information
trimethylammonium chloride (GTMAC),
generating chitooligosaccharides with a qua-
ternary ammonium function (COS-GTMAC)
(Fig. 14.2). The derivatives presented a greater
antimicrobial activity than the non-modificated
oligosaccharides because of the presence of
the quaternary group (Kim
et al
., 2003).
molecular weights smaller than 305 kDa
against
Escherichia coli
(a Gram-negative
bacterium) and
Staphylococcus aureus
(a
Gram-positive bacterium). An increase in
concentration and molecular weight of chi-
tosan oligomers increases the antimicrobial
effect on
S. aureus
. Chitosan oligomers with
a greater molecular weight form a film in the
surface of the microbial cell, preventing the
adsorption of nutrients. In the case of
E. coli
,
a disminution in the molecular weight of
the polysaccharide also increases the anti-
microbial activity. The chitosans of smaller
molecular weight easily enter the external
cell membrane, affecting cellular metabolism.
The preparation by enzymatic depoly-
merization of low molecular weight chi-
tosans in the rank of 4.1-5.6 kDa using
papain from
Carica papaya latex
has been
reported. The antimicrobial activity of the
oligosaccharides obtained was evaluated
against
Bacillus cereus
and
E. coli
, and
presented an antimicrobial effect greater
14.2
Biological Activity
Zheng and Zhu (2003) studied the antimi-
crobial activity of chitosan oligomers with
CH
2
OH
CH
2
OH
O
O
O
O
HO
HO
NHR
NH
2
n
R:H; CH
3
CO
Fig. 14.1.
Chitosan structure.
CH
3
CH
2
OH
O
N
+
Cl
-
CH
2
CH
CH
2
CH
3
O
+
HO
NH
2
n
CH
3
O
Glycidyl trimethylammonium chloride
(GTMAC)
Chitooligosaccharide (COS)
CH
2
OH
O
O
HO
NH
n
CH
3
N
+
Cl
-
CH
2
CH
CH
2
CH
3
OH
CH
3
COS-GTMAC
Fig. 14.2.
Reaction of chitosan (COS) with glycidyl trimethylammonium chloride (GTMAC).
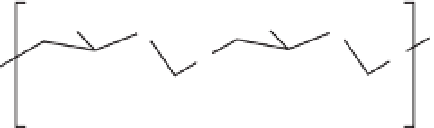






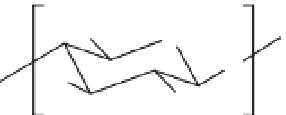


Search WWH ::

Custom Search