Biology Reference
In-Depth Information
biogenic interest (Hahlbrock and Grisebach,
1975) because the biochemical precursor
chalcones and flavanones, 2¢,4¢-dihydroxy-
chalcone, 2¢,4¢-dihydroxy-3¢-methoxy-chalcone,
7-hydroxyflavanone and 7-hydroxy-8-meth-
oxyflavanone, were isolated from the same
source (Pederiva
et al
., 1975). The key steps
in the formation of flavonoids is the conden-
sation, catalysed by chalcone synthase, of
three molecules of malonyl-CoA with an ester
of coenzyme A and of a hydroxycinnamic
acid, as a general rule
p
-coumaryl-CoA (the
incorporation of caffeoyl-CoA seems quite
exceptional, as the extra hydroxylation of the
B ring occurs late in the process). In normal
physiological conditions, chalcone tends to
its cyclization in a reaction catalysed by chal-
cone isomerase, which induces a stereospe-
cific closure of the cycle with formation of
the basic structure of flavanones. To date, the
mechanism of transformation of flavanones
into flavones has not been elucidated.
Moreover, the presence of 2¢,4¢-dihydroxy-
chalcone and 2¢,4¢-dihydroxy-3¢-methoxy-
chalcone were also reported to occur together
in the leaf resin of
Acacia neovernicosa
(Wollenweber and Siegler, 1982).
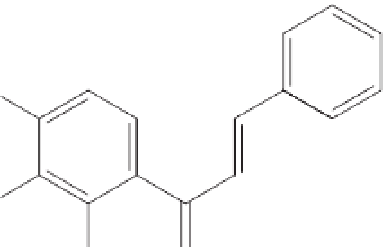
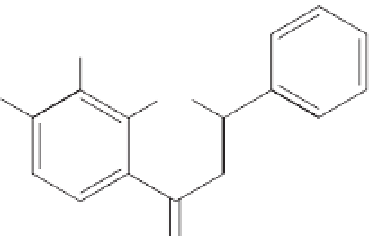
Two caffeic acid esters (Fig. 12.4; Fig. 12.5),
1-methyl-3-(4¢-hydroxyphenyl)-propyl
caffeate (
1
) and 1-methyl-3-(3¢,4¢-
dihydroxyphenyl)-propyl caffeate (
2
) (Svetaz
et al
., 2004), together with 2¢,4¢-dihydroxy-
chalcone, 2¢,4¢-dihydroxy-3¢-methoxy-chalcone
and 7-hydroxyflavanone were isolated from
ethanol extracts of the aerial parts (leaves and
twigs). The chemical synthesis of both caffeic
acid esters was performed (Ramachandra and
Subbaraju, 2006) starting from appropriate
substituted benzaldehydes (Kavitha
et al
.,
1999; Venkateswarlu
et al
., 2006). Hydroxy-
substituted cinnamic acid esters are widely
distributed in the plant kingdom and usually
exist as esters of organic acids or sugars, or are
bound to a protein and other cell wall poly-
mers. Spectroscopy data of the synthetic pro-
pyl caffeates are in good agreement with those
reported for the natural products (Svetaz
et al
., 2004). Synthetic products were, how-
ever, obtained as optically inactive
d
/
l
-isomeric
mixtures, whereas the naturally occurring
compounds are
l
-isomers (
1
, [a]
D
: −27.0° (
c
0.39,
MeOH) and
2
, [a]
D
: −3.65° (
c
0.25, MeOH)).
3
2
4
5
1
HO
6
5
b
6
4
3
1
a
2
R
OH
O
Fig. 12.1.
Chemical structure of
2
¢
,4
¢
-dihydroxychalcone (R = H) and
2
¢
,4
¢
-dihydroxy-3
¢
-methoxy-chalcone (R = OCH
3
).
R
7
O
HO
O
Fig. 12.2.
Chemical structure of
7-hydroxyflavanone (R = H) and 7-hydroxy-
8-methoxyflavanone (R = OCH
3
).
R
HO
O
OH
O
Fig. 12.3.
Chemical structure of
3,7-dihydroxyflavone (R = H) and 3,7-dihydroxy-
8-methoxyflavone (R = OCH
3
).
a new natural product, whereas 3,7-dihy-
droxyflavone (Fig. 12.3) was a known com-
pound reported earlier from
Platymiscium
praecox
(Braga de Oliveira
et al
., 1972).
The isolation of 3,7-dihydroxyflavone
and 3,7-dihydroxy-8-methoxyflavone is of
Search WWH ::

Custom Search