Biology Reference
In-Depth Information
labelling experiments using malonate and
acetate where the acetyl-CoA is the starter
unit. No labelled acetate was detected into
the side-chain moiety of longer chain ARs,
however, suggesting that in such cases a
convergence of the fatty acid and polyketide
pathways occurs (Fate and Lynn, 1996).
In agreement with this explanation, further
research showed that fatty acid units act
as direct precursors to form the side-chain
moiety of alkylresorcinols (Suzuki
et al
.,
2003). In this case, the intermediate
3a
is orcellinic acid-ACP (R = H), which
seems to condense onto a pre-existing fatty
acid unit
1b
, affording a 6-(2′-oxoalkyl)-
resorcinolic acid
7
, as shown in Route B of
Fig. 10.2. The reduction of the 2′-oxo group
to a methylene group affords 6-alkylresorci-
nolic acid
3b
, which can be decarboxylated
to liberate 5-AR.
Research on the biosynthethic pathway
of sorgoleone, an allelopathic quinone syn-
thesized and exuded from root hairs of
Sorghum bicolor
, showed that the lipid tail
and the phenol head of the intermediate
5-pentadecatriene resorcinol are synthe-
sized in different subcellular compartments
(Dayan
et al
., 2003). The 16:3 fatty acid pre-
cursor of the tail is synthesized in the plas-
tids by the combined action of fatty acid
synthase and desaturases. Then, it is
exported out of the plastids and converted
to 5-pentadecatriene resorcinol by a type III
polyketide synthase similar to a stilbene
synthase, because it catalyses the C2 to C7
aldol intramolecular cyclization resulting
in orsellinic acid-type rings. In rye seed-
lings relative high levels of ARs were found
in plastids and mitochondria, suggesting
that these phenolic lipids may be synthe-
sized in such compartments (Deszcz and
Kozubek, 2000).
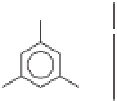
Hydrophobic tail
R
Hydrophilic head
HO
OH
Fig. 10.1.
The general structure of alkylresorcinols
commonly found in cereal grains. R is a linear alkyl
chain that can be saturated, unsaturated and/or
have different degrees of oxygenation.
practically insoluble in water and have very
low critical micelle concentrations, in the
range of 4.5-8.5 mM, varying according to
the tail length and degree of unsaturation of
the homologue considered. The hydropho-
bicity of these compounds is also reflected
in their high values of octanol/water parti-
tion coefficients, which explain their easy
incorporation into the phospholipid bilay-
ers. Plants are able to increase the polarity
of these substances by glycosylation as sug-
gested by the discovery in recent years of
5-n-AR glycosides in the leaves of
Grevillea
robusta
and root exudates from rice seed-
lings (Kong
et al
., 2002; Yamashita
et al
.,
2008). Nevertheless, most 5-n-ARs discov-
ered until today were found as aglycones,
especially in cereal seedlings and grains
(Zarnowsky and Kozubek, 2002).
The biosynthesis of ARs, and phenolic
lipids in general, was predicted through the
polyketide pathway (Vickery and Vickery,
1981). In this interpretation, biosynthesis
starts with a fatty acid unit commonly
known as the acyl-CoA starter unit (Route A,
Fig. 10.2). A polyketide synthase catalyses
the condensation of 3 malonyl-CoA units to
the fatty acyl-CoA starter unit, followed by
second and third extensions of malonyl-
CoAs to 3-oxoacyl-acyl carrier proteins
(3-oxoacyl-ACPs), yielding a polyoxometh-
ylene intermediate
2
in which all the
carbonyl oxygens are retained. An aldol
condensation of the acyclic intermediate
2
and the reduction of the keto groups to
hydroxyl groups yields the intermediate
3a
.
The release of ACP and decarboxilation of
3a
yields a 5-AR
4
with an odd-carbon-number
chain. The liberation of ACP from
3a
affords
a 6-alkylresorcinolic acid
5
. This biosyn-
thetic pathway was suggested for short-chain
ARs and their reduced analogues, based on
10.3
Sources of Alkylresorcinols
The ARs have been found in higher plants,
algae, mosses, fungi and bacteria. In plants
they were detected for the first time in
Ginkgo
biloba
, a gymnosperm tree (Morimoto
et al
.,
1968). Further research showed the presence
of ARs in angiosperm species, including
Search WWH ::

Custom Search