Biology Reference
In-Depth Information
of
K
m
is approximately fourfold higher
than that obtained using a spectrophoto-
metric method. This is in good agreement
with the previously reported observations
(Berry
et al.
, 1997).
Salicylic acid (Machlin and Bendich,
1987) did not inhibit soybean lipoxygen-
ase-1 up to 200 mM, suggesting that a pen-
tadecenyl side chain is essential to elicit
the activity. The pentadecenyl side chain
alone is not enough, however, to elicit the
activity because cardanol (C
15:1
), which
possesses the same side chain as anacardic
acid (C
15:1
), acted neither as a substrate nor
as an inhibitor.
As far as the present cell-free experi-
ment using soybean lipoxygenase-1 is con-
cerned, the inhibition kinetics observed do
not exceed 5 min; however, much longer
observation is needed from a practical point
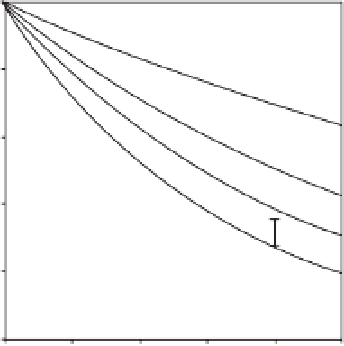
of view. The time course of oxidation of
linoleic acid catalysed by soybean lipoxyge-
nase-1 in the presence of different anacardic
acid (C
15:1
) concentrations is shown in
Fig. 9.5. At each concentration of anacardic
acid (C
15:1
) the rate slowly decreased with
increasing time until a straight line ran
parallel to the x-axis, indicating that the
enzyme activity was lost.
9.7
Conclusion
The oxidative degradation of polyunsatur-
ated fatty acids occurs in two sequential
steps of initiation and propagation
(Svingenn
et al
., 1979). Antioxidative
materials acting in living systems are
therefore classified as preventive antioxi-
dants and chain-breaking ones (Halliwell
and Gutteridge, 1990b). In view of the
present investigation, it seems that the
antioxidant activity of anacardic acids is
not due to radical scavenging but to pre-
venting. They may be advantageous to
suppress the formation of free radicals
and active oxygen species in the first line
of defence. Safety is a primary considera-
tion for antioxidants in food products. In
connection with this, the radical-scavenging
antioxidant traps an active radical and
the antioxidant-derived radical is formed.
The fate of this newly formed radical is
important in determining the total potency
of the antioxidant. For example, several
inhibitors of lipid peroxidation have the
potential to accelerate free-radical dam-
age to other biomolecules (Halliwell
et al
.,
1995). Because of this Janus-like property,
scavenging antioxidants are also known
as a double-edged sword. The data so far
obtained indicate the advantage of ana-
cardic acids as preventive antioxidants. In
addition, the fact that anacardic acids are
known in the cashew apple and nut,
which have been continuously consumed
by people for many years, should give
them another considerable advantage as
antioxidants.
Anacardic acids were previously
reported to have high selectivity toward
transition metal ions, especially Fe
2+
and
Cu
2+
(Nagabhushana
et al
., 1995). The
ability of the high selectivity of chelation
toward Fe
2+
and Cu
2+
of anacardic acids
should give them considerable advantage
1.0
0.8
0
0.6
1
0.4
2
3
0.2
0.0
0
5
10
Time (min)
15
20
25
Fig. 9.5.
Time dependence of the fractional
velocities for the catalysis of linoleic acid soybean
lipoxygenase-1 in the presence of several
concentrations of anacardic acid (C
15:1
). Conditions
were: 0.1M sodium borate buffer, pH 9.0, linoleic
acid 30
m
M and 0.188
m
g/ml soybean
lipoxygenase-1. Concentrations of anacardic acid
(C
15:1
) for curves 0-3 were 0.8, 2, 4 and 6
m
M,
respectively.



















Search WWH ::

Custom Search