Agriculture Reference
In-Depth Information
Fig. 3.9
Concentration-response curve for monoammonium glycyrrhizinate in water.
Redrawn using the data of DuBois
et al.
(1991).
1990). Crude extract powder and the refi ned ammoniated glycyrrhizin products
are preferred for confectionery and pharmaceuticals.
The US FEMA GRAS list permits liquorice extracts and ammoniated
glycyrrhizin in a wide range of products, including baked goods, frozen dairy
products, beverages, confectionery and chewing gum and even meat products (not
the ammoniated salt).
Regulatory status
The base products monoammonium glycyrrhizinate and ammoniated glycyrrhizin
are approved for use in foods in the US, Europe, Australia, China and India, and
numerous other countries throughout the world.
As well as a sweetener, liquorice extracts and glycyrrhizin are FEMA GRAS
for a wide range of fl avouring uses in the US. Liquorice and its derivatives are
also generally regarded as safe (GRAS) by the FDA (21 CFR 184.1408) within
specifi ed limits and for use as a fl avour enhancer, fl avour and, in beverages, as a
surfactant.
Glycyrrhizinic acid and its ammonium salt are included in the EU register of
fl avouring substances. However, concerns about the pharmacological effects of
glycyrrhizin have led to the imposition of special labelling requirements (EC
2004) to warn consumers of glycyrrhizin contents above either 100 mg/kg in
confectionery or 10 mg/l in beverages. Signifi cantly greater contents trigger more
explicit warnings for consumers with hypertension.
The EU Scientifi c Committee for Food (SCF) declined to set an ADI for
glycyrrhizin owing to inadequate toxicological information, but recommended




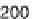

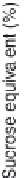
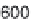


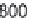
























Search WWH ::

Custom Search