Agriculture Reference
In-Depth Information
Unfortunately, there is no agreement on a single sucrose equivalent
concentration at which to quote potency. Comparison of the potency of sweeteners
is thus diffi cult, despite the apparently ready availability of fi gures. The best
practice is to furnish a concentration-response curve, usually from zero
concentration of sweetener up to around the plateau value. Alternatively, quoting
a potency and identifying the sucrose equivalence at which it was measured is
helpful. Sadly, neither of these approaches is common. Consequently, quoted
potency values for HPS are often only the vaguest of guides to the relative
sweetening power of the substance concerned in practical applications.
Sugars and polyols, on the other hand, exhibit substantially fi xed potencies
over a wide range of concentrations. That is to say, the graph of sweetness against
concentration is not a curve as for HPS, but a straight line (DuBois
et al.
1991).
Accordingly, the potency of sugars and polyols is usually accurately described by
a single number.
3.2
Commercial bulk low-calorie sweeteners
3.2.1
Erythritol
Structure, source
Erythritol is a monosaccharide polyol (as are sorbitol, xylitol and mannitol). It
occurs naturally in minor amounts in some fruits (watermelon, pear and grape)
and fermented foods such as soy sauce, cheese, wine and beer. It is manufactured
by fermentation using yeast-like fungi such as
Trichosporonoides megachiliensis
or
Moniliella pollinis
. The fermentation broth is heated to kill the production
organism and dead cells are removed by fi ltering. Once erythritol is separated
from the fermentation broth, it is purifi ed by ion exchange resin, activated
charcoal, ultrafi ltration and crystallisation. The fi nal crystalline product is more
than 99% pure (SCF 2003a).
Physico-chemical properties
Erythritol is a crystalline, white, anhydrous, non-hygroscopic solid that has very
much the appearance of table sugar. Chemically, erythritol is (2
R
,3
S
)-butane-
1,2,3,4-tetraol (Fig. 3.1), a linear, four-carbon polyol. It is non-reducing and,
therefore, does not undergo Maillard browning. It is stable to acid hydrolysis and
to high temperatures. It melts at 121°C.
Erythritol is moderately soluble and 37-43 g will dissolve in 100 g water at
25°C (Embuscado and Patil 2001). This is relatively low (only mannitol and
Fig. 3.1
Structure of erythritol.

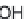




Search WWH ::

Custom Search