Agriculture Reference
In-Depth Information
[10.42] [10.38]
10.7
Assessing the natural status of aroma chemicals
10.7.1
Hi-tech methods
Measurement of
14
C level
The measurement of the
14
C level of an aroma chemical of plant origin
gives the date that the carbon in the molecule was last in the atmosphere. This
is
de facto
radiocarbon dating, making use of the fact that the upper
atmosphere has a constant 'steady state' concentration of
14
C, a balance
between its formation from
14
N by impact of cosmic rays and its subsequent
decay back to
14
N over a half-life of 5700 years. The measurement is usually
carried out by traditional scintillation counting methods, and indicates whether
the material is 'of recent biological origin' or whether it contains carbon of fossil
origin, in which essentially all the
14
C has decayed. While this only shows the
source of the carbon and says nothing about the methods used to prepare the
material, it remains a good test for materials which are usually isolates; for
example, cinnamaldehyde [10.16] is usually isolated from cassia oil, and the
14
C
test would generally confi rm its natural status. In theory, it could be prepared
from natural benzaldehyde [10.18] by 'chemical' means, but this would not be
economic - a greater concern would be chemical conversion of cinnamaldehyde
to benzaldehyde!
[10.16]
[10.18]
In some cases, however,
14
C would give little or no information about natural
status. For example, furfural [10.43] is produced from cereal (carbohydrate) waste
and hence would pass as natural by the
14
C test, as would any of the materials into
which it is converted without the addition of other carbon atoms. It is hydrogenated
to yield furfuryl alcohol [10.44] and 2-methylfuran [10.45], with the latter a
precursor to 2-methyl-3-furanthiol [10.46]; all of these would appear 'natural' by
radiocarbon testing!






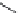

























Search WWH ::

Custom Search