Agriculture Reference
In-Depth Information
compounds increases their effi ciency and thereby reduces the cost in use due to
the lower quantities needed.
9.3.3 Proteins as emulsifi ers and foaming agent
Proteins are generally applied in food products to stabilize emulsions or foams
(Huppertz 2010; Wierenga and Gruppen 2010). This functionality originates from
the amphiphilic nature of specifi c proteins. The term amphiphilic is used for
compounds/molecules that show both hydrophilic (water-loving) and lipophilic
(fat-loving) properties. Due to this bifunctionality, amphiphilic molecules
assemble on the interface between water and hydrophobic materials (air or oil).
One specifi c protein known for its amphiphilic character is
β
-casein (
β
CN).
The
β
-casein molecule is composed of hydrophobic and amphiphilic sequences.
As such the molecule shows good emulsifying properties, and by using enzymatic
hydrolysis, the amphiphilic and hydrophobic stretches can be separated. Two
different amphiphilic peptides can be identifi ed (Caessens
et al.
1999a,b). The
two peptides differ in the length of their negatively charged tail (Fig. 9.5). This
end of the amphiphilic peptide (amino acid residues 1-28) contains four of the
fi ve phosphoserine residues present in
β
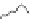
-casein. As depicted in Fig. 9.6 the length
of this negatively charged tail determines the stability of an emulsion prepared
Fig. 9.5
Molecular structure of the protein
β
-casein and peptides derived thereof.
Copyright NIZO, 2012.
Fig. 9.6
Impact of the molecular structure of
β
-casein peptides and the impact on
emulsion stability. Copyright NIZO, 2012.
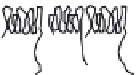
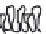
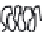




























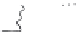





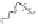

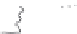














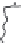





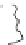























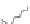
































Search WWH ::

Custom Search