Agriculture Reference
In-Depth Information
Fig. 5.10
Tocopherols' functionality.
concentrations are avoided (Jacobsen
et al.
2008) and fi sh fi llets (Sant'Ana and
Mancini-Filho 2000).
5.3.2 Ascorbic acid
Ascorbic acid is technically a sugar acid, discovered early in the twentieth century,
with
L
-ascorbic acid being the physiologically essential vitamin C.
Ascorbic acid is a natural, water-soluble food antioxidant, distinguished
because of its complexity of modes of action. Ascorbic acid possesses an intriguing
functionality: it can be a metal chelator, an oxygen scavenger and a reducing
agent, and can cause prooxidant or antioxidant effects depending on the system
and the circumstances in which it is used (Frankel 1996). One of the mechanisms
proposed to explain its oxygen-scavenging functionality is based on the
consumption of oxygen and production of water as ascorbic acid is converted to
dehydroascorbic acid (Cort 1974) (Fig. 5.11).
Ascorbic acid is widely used as an oxygen scavenger and synergist in numerous
food applications. As a synergist, it is used to regenerate phenolic antioxidants
that have already donated one or more hydrogens to a more reactive free radical.
This functionality is due to the fact that ascorbic acid has a higher oxidation
potential (greater reducing capacity) than most phenolic antioxidants. It acts
synergistically with tocopherols via the regenerative reduction of the tocopheryl
radical. In this manner, it allows for lower levels of tocopherols to be used.
The benefi cial use of ascorbic has been established for the stabilization of beer
(Wales 1956) and other food applications where it can serve to reduce the oxygen
from the headspace of a closed system (Cort 1974).




































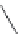






















































Search WWH ::

Custom Search