Agriculture Reference
In-Depth Information
regenerators. Although some antioxidants operate in more than one category, they
are mainly classifi ed based on their interference with various stages of the
antioxidant mechanism (Fig. 5.2):
•
Primary antioxidants: Also known as radical scavengers or chain-breaking
antioxidants, these are substances that can donate a hydrogen atom or an
electron to a radical, and thereby disrupt its ability to continue the free radical
chain propagation process. By donating a hydrogen atom or an electron, the
oxidized antioxidant becomes a radical species itself, stabilized by resonance,
and therefore suffi ciently stable so as to be unable to further oxidize substrates.
Primary antioxidants (both synthetic and natural) are typically phenolic
compounds, such as BHA and BHT, propyl gallate, tocopherols, tea catechins,
or carnosic acid and rosmarinic acid - natural phenolics found in rosemary.
•
Chelators: Also known as metal sequestrants or metal deactivators, these are
substances that bind with metals and prevent them from initiating radical
formation. Examples include ethylenediaminetetraacetic acid (EDTA), citric
acid, phytic acid and phosphoric acid. The key property desired in an antioxidant
is to prevent the cycling of the transition metal ions between its oxidation
states.
Fig. 5.2
Types of antioxidant.

















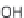









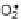










































































Search WWH ::

Custom Search