Environmental Engineering Reference
In-Depth Information
concentration produced a greater yield of HO but a reduced yield of WSO, while resulting in
negligible change in the formation of total oil products. The presence of 0.1M K
2
CO
3
dramatically enhanced organic conversion leading to a low yield of char, while the use of the
K
2
CO
3
suppressed the formation of both types of oils. The use of the two alkaline earth
metals catalysts, i.e., Ca(OH)
2
and Ba(OH)
2
, did not alter biomass conversion significantly,
but catalyzed the formation of WSO and produced much higher yields of total oil products.
The liquefaction atmosphere (inert or reducing) was found to be another important factor
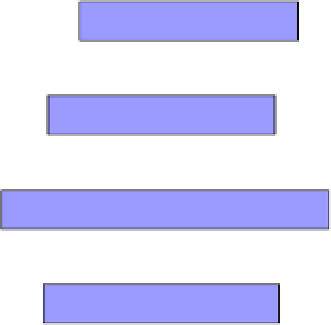
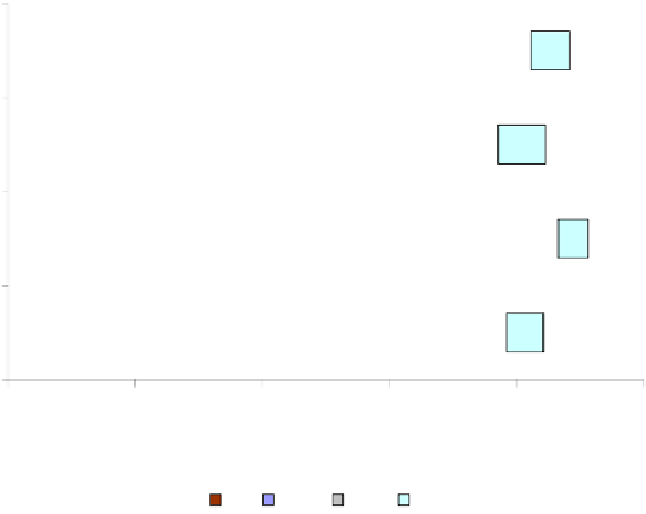
influencing the liquefaction process. As shown in Figure 7, it was demonstrated that the
reducing atmosphere (i.e., H
2
) in the liquefaction process promoted the HO formation while
suppressing the WSO formation. With the presence of 0.1M Ca(OH)
2
and 2MPa H
2
,
liquefaction of the sludge powder in water at 280
o
C for 60 min produced a high yield of HO
(26 wt%), almost two times as high as that in N
2
(13.6 wt%), and it produced total oily
products (HO+WSO) at a yield as high as 60 wt%.
Ca(OH)2
26.0
34.2
22.0
6.2
Liquefaction in H
2
None
21.0
35.6
20.6
7.3
Ca(OH)2
13.6
51.4
21.5
4.8
Liquefaction in N
2
None
5.7
20.3
36.9
21.3
0
20
40
60
80
100
280
o
C, 60 min
9.1 wt% biomass
Initial gas pressure: 2 MPa
Yield (wt%, daf)
HO
WSO
Char
Gas
Figure 7. Variation in the product yields with different liquefaction atmospheres (N
2
and H
2
) (adapted
from Xu and Lancaster, 2008).
In Xu and Lancaster's (2008) work, the energy output/input ratios, calculated based on
Eq. (1), were all <1.0, suggesting that the liquefaction operation tested, in a batch reactor, was
energy inefficient. However, the energy efficiency can be improved by employing a flow-type
reactor and installing a heat exchanger to pre-heat the reactor feed stream using the hot
product stream as well as by combusting the resulting chars/gases to provide a portion of the
heat for the process. Liquefaction of sludge in H
2
, irrespective as to whether a catalyst was
present or not, resulted in significantly improved net energy efficiencies. However, the
operation in H
2
with the presence of Ca(OH)
2
catalyst dramatically enhanced the efficiency to
as high as 0.76.