Chemistry Reference
In-Depth Information
In addition to heat transfer, there are many other scale-up considerations
from a safety perspective. Flammable solvents and gases present a risk
because of the possibility of sparks in a plant environment. When possible,
they should be avoided. If not possible, precautions need to be taken. Every
effort needs to be taken to contain the chemicals. This is especially important
for the safety of workers but also to protect the environment. Serious
consideration needs to be given to generated waste. It should be minimized
but at the very least there needs to be a responsible plan on how to treat it.
Material of construction is a topic that chemists may not be used to
thinking about. In the laboratory, reactions are run in glassware and there are
rarely issues. Plant equipment is often carbon steel or stainless steel. If your
reaction generates or uses a corrosive material such as aqueous HCl, then
there are major issues and this equipment should not be used. For reactions
where steel is not suitable some alloys such as Monel or Hastelloy may be
suitable. Sometimes nickel is used. More commonly, a steel reactor is lined
with glass, effectively making this a glass reactor. Sometimes the glass lining
can have chips or holes due to thermal shock, abrasion or even dropping
tools in a reactor. The glass lining can be tested for thickness by using a
magnetic induction instrument. Defects can be found by conducting a spark
test. In special circumstances, reactors are lined with Teflon.
Sometimes the material of construction is a factor in the reaction. Consider
the free radical chlorination of toluene to make benzyl chloride.
CH
3
CH
2
-Cl
Light
+ HCl
+ Cl
2
Toluene
Benzyl chloride
The reaction should not be done in a steel reactor because of corrosion
issues. But there is another reason. Even the slightest bit of corrosion in the
presence of chlorine will give iron (III) chloride. This serves as a Lewis
acid and catalyzes electrophilic aromatic substitution. The reaction is drawn
showing the formation of the para isomer but other isomers are also formed.
Ring-chlorinated toluene is an unwanted and difficult-to-remove impurity if
you are making benzyl chloride.
CH
3
CH
3
FeCl
3
+ Cl
2
+ HCl
Toluene
Cl






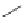















Search WWH ::

Custom Search