Chemistry Reference
In-Depth Information
significant cost improvement versus corn-based ethanol and are planning
two plants in China, each with 400,000 metric ton capacity and at a cost of
$300 million. They have also announced the construction of a technology
development plant in Clear Lake, Texas.
In the Fischer - Tropsch synthesis, carbon monoxide reacts with hydrogen
and is reduced to a hydrocarbon.
H
CH
2
H + n H
2
O
n CO + (2n+1)H
2
n
The reaction is done with a metal catalyst. Catalysts based upon iron
or cobalt have been used commercially for hydrocarbon synthesis [5]. The
mechanism involves adsorption of hydrogen and carbon monoxide on the
metal surface [6]. The Fischer - Tropsch process enables natural gas to be
converted to liquid synthetic fuel. First, the natural gas is oxidized to syn
gas which is then converted by the Fischer - Tropsch process to the liquid
hydrocarbon mixture, which is useful as fuel.
Hydroformylation involves the reaction of an alpha-olefin with carbon
monoxide and hydrogen and a catalyst to form the aldehyde. Common
catalysts are based upon cobalt or rhodium. The reaction is also referred
to as the Oxo process or the Roelen reaction. The reaction is illustrated
below with propylene as the alkene. The linear: branched isomer formation
depends on catalyst selection with most companies trying to maximize the
n-butyraldehyde because it has more uses. However the desired production
ratios vary with market demand for end-use products.
O
+
+CO+H
2
H
CHO
Isobutyraldehyde
Butanal
The aldehydes can be the desired product but often they are reduced to
the alcohol or oxidized to the carboxylic acid. Other reactions, such as aldol
condensations, can be employed as illustrated with butanal. Reduction of the
aldol product gives the commodity chemical, 2-ethylhexanol, used to make
plasticizers such as dioctyl phthalate (dioctyl phthalate is the common name
for the diester of phthalic acid with 2-ethylhexanol, but more precisely, it can
be called di-2-ethylhexyl phthalate).
H
O
O
O
O
H
2
Aldol
H
H
+
H
H
H
2-ethylhexanol
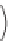


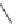





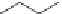















Search WWH ::

Custom Search