Chemistry Reference
In-Depth Information
used as a mixture of enantiomers. Similarly difenoconazole and epoxicona-
zole have asymmetric carbons and are used as a mixture of stereoisomers. In
the case of epoxiconazole, the chlorophenyl group is on the same side of the
epoxide as the triazolomethyl substituent.
Triazole ring
N
N
N
N
N
N
O
Cl
N
N
N
Cl
OO
Cl
O
HO
Cl
F
Epoxiconazole
Difenoconazole
Tebuconazole
QoI (quinone outside inhibitors) fungicides act at the Quinol outer binding
site of the cytochrome bc1 complex thereby inhibiting respiration and caus-
ing death of the fungus. Strobilurins such as azoxystrobin and pyraclostrobin
dominate this class of fungicides. In 2010, Syngenta sold $1 billion of azoxys-
trobin under the trade name Amistar [65]. The term strobilurin derives from
Strobilurustenacellus
, a fungus from which these compounds were originally
isolated.
NN
O
N
O
Cl
N
CN
O
O
O
N
OCH
3
CH
3
O
O
H
3
C
H
3
C
Pyraclostrobin
Azoxystrobin
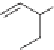
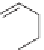
One class of seed treatment fungicides is the carboxanilides; they are
amides of aniline. Carboxin, mepronil, and flutolanil are all commercial
members of this class [66]. They are in group C2 and are succinate
dehydrogenase inhibitors.
O
CF
3
O
CH
3
H
H
O
CH
3
N
N
H
N
O
S
O
O
Carboxin
Mepronil
Flutolanil






























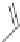

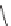





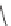









































































Search WWH ::

Custom Search