Chemistry Reference
In-Depth Information
Cl
Cl
N
N
CH
2
OH
N
CO
2
H
N
N
N
N
N
HN
N
N
N
K
+
Losartan
Active metabolite of losartan
The group name sartan was coined and other sartans were developed.
Several, such as valsartan (Diovan
®
, Novartis) and irbesartan (Avapro
®
,
Bristol-Myers Squibb) retained the biphenyl substituted tetrazole ring of
losartan.
O
N
CO
2
H
O
N
N
N
HN
N
N
HN
N
N
N
Valsartan
Irbesartan
The synthesis of losartan has been the subject of considerable research. The
various syntheses have been discussed [25] in regards to their patent position
and their process friendliness or ability to scale. In an early synthesis, the
tetrazole is formed by reacting the substituted benzonitrile with sodium azide
in a 1,3-dipolar cycloaddition. By protecting the tetrazole as the trityl deriva-
tive, the product is soluble in toluene and unreacted azide can be removed by
washing with aqueous base. The use of a bulky trityl group also alleviated
concerns with exothermic decomposition of the unprotected tetrazole. Ben-
zylic bromination under radical conditions with N-bromosuccinimide was
followed by displacement of the bromide with the substituted imidazole. The
aldehyde is reduced to the alcohol, the trityl group cleaved under acidic con-
ditions and then treatment with KOH gives losartan.
Br
Cl
CH
3
CH
3
H
2
C
1. NaN
3
/Bu
3
SnCl
2. NaOH
3. Ph
3
CCl
N
CHO
CPh
3
CPh
3
Bu
H
NaBH
4
NBS
AIBN
N
N
N
N
N
N
N
N
CN


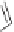














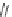












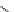
























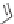






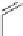































































Search WWH ::

Custom Search