Chemistry Reference
In-Depth Information
Lovastatin can be prepared by a fermentation process
15
in the presence
of a specific microorganism. Lovastatin can be converted to simvistatin
16
.
Hydrolysis of the ester followed by reclosure of the lactone gives the diol.
The less-hindered alcohol can be selectively protected using the bulky
t-butyldimethylchlorosilane. The free alcohol can be esterified by the acid
chloride in the presences of dimethylaminopyridine acylation catalyst. The
silyl ether can be selectively removed by treatment with tetrabutyl ammo-
nium fluoride. The fluoride anion reacts at the silicon without hydrolyzing
the lactone or ester.
HO
HO
O
O
O
O
O
H
Si
O
O
Cl
1. LiOH
(aq)
CH
3
CH
3
Fermentation
2. H+/heat
H
3
C
H
3
C
Lovastatin
Si
O
O
HO
O
Si
O
O
O
O
O
O
O
O
H
O
O
Cl
O
Bu
4
N
+
F
−
Acetic acid
CH
3
CH
3
CH
3
DMAP
H
3
C
H
3
C
H
3
C
Simvistatin
Another treatment for cholesterol is niacin. The use of niacin predates the
statins. Niacin is also known as nicotinic acid or vitamin B
3
. The name, niacin
comes from nicotinic acid and vitamin and was coined to avoid confusion
and so that people would not think that the vitamin contained nicotine or
that tobacco products contained vitamins. Niacin inhibits lipoprotein synthe-
sis by preventing the secretion of very low density lipoprotein from the liver.
Very low density lipoprotein is a precursor of low density lipoproteins (LDL).
However there are several adverse side effects with niacin including flushing,
warm skin, itching rash, constipation, nausea, heartburn, and problems with
liver function. Because of these side effects, niacin is often used in a controlled
release form [17] and even in this form is unsuitable for many patients.
CO
2
H
N
Nicotinic acid


























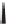




















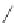
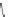
































































Search WWH ::

Custom Search