Chemistry Reference
In-Depth Information
A method of treating asthma with optically pure R(
−
) enantiomer has been
patented [32].
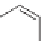
OH
N
HO
OH
R(
−
)-albuterol
Another example is propoxyphene [33]. The (2R,3S)-enantiomer, sold as
the hydrochloride and once marketed as Darvon
®
, is an analgesic. The 2S,3R
enantiomer is an antitussive and was once marketed as Novrad
®
. Today, nei-
ther is marketed in the United States. Darvon
®
was removed in 2010 and
Novrad
®
removed about thirty years earlier. The scientists at Lilly who dis-
covered these compounds were clever in their naming. NOVRAD is the mirror
image of the name, DARVON.
O
H
H
3
C
O
N
2R,3S-propoxyphene
Darvon
The practice of patenting a single enantiomer in a previously known effec-
tive racemate is sometimes called a “chiral switch.” Theoretically you could
imagine that a pharmaceutical company that invented a new active drug could
patent the racemic mixture and obtain exclusivity for 20 years. At the end
of the 20 years, they could patent the more effective enantiomer and gain
another 20 years for a total of 40 years of exclusivity. However in practice, this
does not happen today. A pharmaceutical company that invents a new chiral
active will investigate the enantiomers and attempt to obtain patent protection
for both the racemate and the effective enantiomer. Otherwise, they risk the
possibility that another company might patent the more effective enantiomer
and block the first company from practicing the best form of the drug. As an
example, the patent for using optically pure R (
−
) albuterol was not by the
original company that discovered albuterol.
The majority of racemic pharmaceuticals have one major bioactive
enantiomer. This is called the eutomer. The other inactive or less active
enantiomer is called the distomer [34]. A number of cardiovascular drugs













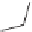













Search WWH ::

Custom Search