Chemistry Reference
In-Depth Information
6. Data
should
be
acquired,
processed
and
archived
to
ensure
data
integrity.
Because the pharmaceutical industry is a global industry, there is a need
for regulations beyond the United States. The International Conference on
Harmonization of Technical Requirements for Registration of Pharmaceuti-
cals for Human Use (ICH) began in 1990. It is a project that brings together
the regulatory authorities of Europe, Japan, and the U.S. and experts from the
pharmaceutical industry in the three regions to discuss scientific and techni-
cal aspects of product registration. The purpose is to make recommendations
on ways to achieve greater harmonization in the interpretation and appli-
cation of technical guidelines and requirements for product registration in
order to reduce or obviate the need to duplicate the testing carried out during
the research and development of new medicines. ICH guidelines represent
agreed-upon scientific guidance for meeting technical requirements for reg-
istration within the three ICH regions - EU, U.S., and Japan. ICH publishes
guidelines on a variety of topics related to the pharmaceutical industry. For
example, there are documents on stability testing, on validation of analytical
procedures, on impurities in new drug substances, and on many other subjects.
10.3 SYNTHETIC CONSIDERATIONS
Most pharmaceuticals are small synthetic molecules, most of which are based
upon nitrogen heterocycles. These molecules are typically made by multi-step
syntheses. The best synthesis is one that gives the desired compound with
the best quality and the lowest cost. Often lowest cost is determined by the
number of steps, the yield of each step, and the complexity of each step. It is
important for pharmaceutical companies to develop attractive syntheses and
considerable resources are expended in process development.
As an example, consider the drug losartan K (Cozaar), which is commonly
prescribed for hypertension. The drug was invented by Dupont workers and
developed and marketed with Merck & Co. Inc. The patent [25] covering the
composition expired in 2009 and the drug is now offered in generic form.
Several early syntheses originated from 4
′
-methyl-biphenyl-2-carbonitrile.
N
Cl
N
CH
3
OH
N
K
+
N
−
N
CN
N
Iosartan K


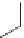







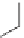












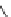








Search WWH ::

Custom Search