Chemistry Reference
In-Depth Information
O
O
O
O
O O
O
O
Benzoyl peroxide
Di-t-butylperoxide
Dicumylperoxide
Peroxides can be used to promote crosslinking of a rubber as is done
in dynamic vulcanization. Dynamic vulcanization is usually done in an
extruder and is the process of selectively crosslinking rubber during its melt
mixing with a molten thermoplastic such as polypropylene. This produces
thermoplastic vulcanates, often referred to as TPVs [16]. Peroxides are
also used to provide grafting reactions. For example, polypropylene, maleic
anhydride, and a peroxide initiator in a twin-screw extruder give a graft
polypropylene [17]. These polymers can have improved properties such as
improved adhesion.
O
O
O
Polypropylene +
O
+
Grafted polypropylene
O
O
O
Polyethylene can be crosslinked with peroxides to make it more suitable for
coatings for electrical power cables [18]. Peroxides are also used to crosslink
(also called cure) thermoset polyesters. Thermoset polyesters have a reactive
alkene and are used to make fiberglass compositions such as used for boat
hulls. Variations are possible, but commonly they are copolymers of fumaric
acid or maleic anhydride with phthalic acid or isophthalic acid and a diol.
The oligomer is typically dissolved in styrene. When a peroxide is mixed
with the solution, crosslinking occurs and the polyester cures. The styrene
solvent takes part in the reaction and the solution hardens.
CO
2
H
O
OH
+
O
+
HO
CO
2
H
O
Propane diol
Isophthalic acid
Maleic anhydride
O
O
O
O
O
O
O
x
y
O
Oligomeric unsaturated polyester copolymer

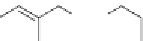
































































Search WWH ::

Custom Search