Chemistry Reference
In-Depth Information
and Fortron
®
(Fortron Industries, a joint venture of Hoechst Celanese and
Kureha Chemical).
Cl
Cl
+ Na
2
S
S
n
PPS
PPS is particularly well suited to demanding applications where it must
stand up to high temperatures and solvent attack while maintaining over-
all mechanical and dimensional integrity. PPS has continuous use tempera-
tures exceeding most other thermoplastics. It has a low coefficient of thermal
expansion, good inherent flame retardance and good electric properties. One
drawback of PPS is that it is relatively brittle. It is usually compounded with
glass or carbon fibers or with mineral fillers to improve its impact strength and
other mechanical properties. Because of its thermal stability and resistance to
automotive fluids, filled PPS has many applications in automotive parts such
as switches, alternators and fuel pump housings.
8.11 ACETAL RESIN
Acetal resin has a good combination of strength and stiffness, good creep
resistance, good fatigue resistance, abrasion resistance, and low wear and fric-
tion. Creep is the tendency of a material to deform under load. For example,
a plastic grocery bag made of polyethylene may not stretch if it has a heavy
object in it. However, if suspended for a period of time it may permanently
stretch and deform. This is more likely to happen as temperature increases.
Creep is deformation as a function of time for a sample under constant stress.
Fatigue is the progressive damage that occurs when a material is subjected
to cyclic loading. Consider a piece of uncoated copper wire. If you bend it,
it doesn't break. However, if you repeatedly bend it back and forth, it will
eventually break. This is an example of fatigue. Fatigue resistance is impor-
tant for a material that is repeatedly flexed back and forth. Creep resistance is
important for a material, such as a hanging strap, that is constantly subjected
to a load. Because of its properties, acetal resin is often used for moving parts
such as gears or zippers.
Acetal resin is either a homopolymer or copolymer of formaldehyde. The
homopolymer is sometimes called polyoxymethylene or POM. It is made
by polymerizing either anhydrous formaldehyde or its cyclic trimer triox-
ane. The polymerization can be done using either acidic or basic catalysts.
At least some of the end groups are hydroxyl groups and with heat, the resin

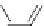












Search WWH ::

Custom Search