Chemistry Reference
In-Depth Information
+
O
HO
OH
O
O
HO
2
C
CO
2
H
O
O
O
x
The second largest volume polyester is polybutylene terephthalate, PBT,
made from dimethyl terephthalate and 1,4-butanediol with a titanium alkox-
ide catalyst. Butanediol can be prepared from acetylene and formaldehyde
followed by reduction of the triple bond.
H
H
H
H
O
H
H
+2
Formaldehyde
O
H
H
C
C
H
O
H
OH
HO
1,4-butanediol
Acetylene
O
O
CH
3
O
O
O
H
+ 2x CH
3
OH
+
H
O
O
O
H
3
C
O
x
O
DMT
Because the longer butanediol imparts a little less rigidity and more
flexibility than ethylene glycol, PBT has a lower T
m
and T
g
than PET. The
extra molecular motion due to the butanediol is also a reason why PBT tends
to crystallize faster than PET; a big advantage in injection molding. For PBT,
T
m
is about 220
∘
CandT
g
is about 40
∘
C. PET is about 30
∘
C higher for
each value. Changes in the carboxylic acid portion also influence thermal
properties. Going to the more rigid, polyethylene naphthalate increases the
T
m
by about 20
∘
C versus PET. The more flexible polyethylene adipate
decreases the T
g
and T
m
, each by more than 100
∘
C versus PET.
PBT has many applications, most of them being injection molded articles.
PBT is a crystalline polymer and has excellent solvent resistance. It can be
suitable for applications requiring heat capabilities approaching its melting
point of 220
∘
C. When blended with glass fibers, PBT can be injection
molded to give articles with excellent stiffness. These blends are used for
many electrical applications such as connectors. PBT is also blended with
other polymers to impart solvent resistance to the final article. One example
of this is automobile bumpers with the PBT imparting gasoline resistance.
Common tradenames for PBT are Valox
®
(SABIC) and Ultradur
®
(BASF).
Both PET and PBT are made by a bulk melt process, typically in con-
tinuous fashion. Using PBT as an example, dimethyl terephthalate and an
excess of butanediol are combined at a temperature above the melting point
of DMT, typically around 150 - 160
∘
C in the presence of a tetraalkyltitanate.
The reaction progress can be monitored by the distillation of methanol. As



















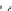
































Search WWH ::

Custom Search