Chemistry Reference
In-Depth Information
contaminates subsequent batches and can cause defects. The flakes are often
less soluble and darker. These imperfections are particularly noticeable in film
applications where they cause optical defects due to gels. These film defects
are called “fisheyes.” To minimize this, reactors are periodically cleaned. For
this reason, most PVC processes are done batchwise or semi-continuous and
not in true continuous fashion like polyethylene or polypropylene. At one
time, PVC adhering to polymer walls was such a problem that people had to
go inside the reactors and manually clean them. Process improvements have
since been made including a discovery about coating of reactor surfaces to
minimize adhesion [3].
In the case of VCM, the head of the monomer unit is arbitrarily designated
as the chlorine-containing carbon and the tail as the non-chlorine containing
carbon [4]. Because the radical intermediate is more stable when the carbon
has a chlorine atom, the monomers assemble in an almost exclusively head to
tail fashion.
H
H
H
H
H
Cl
H
Cl
H
Cl
Cl
.
Head to tail
more stable radical formed
R
R
C
.
C
C
H
H
H
H
H
H
H
Cl
H
Cl
H
Cl
H
.
Head to head
less stable radical
R
R
C
.
C
C
H
Cl
Although most of the PVC is an assemblage of VCM in a head to tail
fashion, there are different types of structural defects which have been found
in PVC. Because of these defects, PVC has poorer thermal stability than
would be otherwise expected. Thermal degradation occurs at one of these
weak spots and the polymer can eliminate HCl from the neighboring two
carbons. The resultant olefin destabilizes the neighboring bonds and another
HCl molecule is eliminated, with eventually, HCl continuing to “unzipper”
all along the polymer chain. Two defects thought to cause much of this
degradation are allylic chlorines and chlorines on tertiary carbons [5].
Because of this tendency towards HCl elimination, stabilizers are neces-
sary for PVC. There has been a large amount of research in this area. Lead
stabilizers are effective but are no longer used to any extent in the U.S. When
some imported toys are found to have lead contamination, if it is not from the
paint or pigments, it can be from PVC made with lead stabilizers. For some
time in the U.S., tin-based stabilizers have been widely used. Compounds
such as dibutyltin bis (isooctylthioglycolate) are effective.



























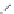






Search WWH ::

Custom Search