Chemistry Reference
In-Depth Information
crystalline polymers have melting points (often abbreviated as T
m
). However,
unlike small molecules, polymers are never completely crystalline. Because
the regions that can pack together are present within a chain and because
of chain entanglement, there is a limit to how much of the polymer chain
can be ordered and be in crystal form. A crystalline polymer is never 100%
crystalline and depending on the polymer, values of 50 - 95% crystallinity are
common. The T
m
of a crystalline polymer determines its maximum upper-use
temperature. Above the T
m
, the polymer softens and does not retain its
physical properties.
Not all polymers are crystalline. For these amorphous polymers it is the
glass transition temperature (T
g
) that determines the upper-use temperature.
An amorphous polymer is said to be in the hard, glassy state when it is at a
temperature below the T
g
. At this temperature, there is only localized motion
of the polymer. Bonds are stretching and wagging, but the molecules remain
in place. As the temperature is increased, the thermal energy is transferred
to kinetic energy and the motion increases. Concurrent with the increase in
localized motion, is an increase in volume. This is due to vibrational motion,
the bending, stretching, and wagging of bonds. As the temperature continues
to increase, at a certain temperature, the T
g
, there is enough energy to enable
longer range motion of the molecules. They are no longer “frozen” in place
but can slide by each other. Above the T
g
, the polymer becomes soft. The
volume continues to increase with increasing temperature, but at a different
rate. The T
g
defines the point at which there is a change in slope of the
volume/temperature curve. A crystalline polymer has both a T
g
and a T
m
.
The T
g
is at a lower temperaturebut it is the T
m
that determines the upper use
temperature.
QUESTIONS
1.
If styrene costs 42.0 ¢ per pound and the price of the catalyst is negligible,
assuming 100% yield calculate the raw material cost of polystyrene to the
nearest tenth of a cent.
2.
Label (name) each of the following to indicate the stereochemistry.





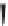







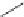




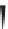


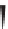
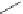









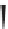














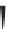









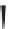



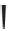














































Search WWH ::

Custom Search