Chemistry Reference
In-Depth Information
+ BCl
4
−
+ BCl
3
Cl
x
x
Because of problems with termination due to radical coupling and radical
disproportionation reactions, living radical polymerizations were invented
much later than living cationic polymerizations which in turn were invented
three decades after living anionic polymerizations. Strictly speaking, they are
not living because there is not an absence of irreversible termination. Rather,
as in the quasi-living cationic polymerizations, there is an equilibrium
between the propagating polymer radical and a dormant polymer chain.
The term, “reversible-deactivation radical polymerization, RDRP” has been
proposed [21] for these radical polymerizations. Different names have been
given to these polymerizations depending on the nature of the dormant
chain. For example, the dormant chains may be alkyl halides, as in atom
transfer radical polymerization (ATRP), thioesters, as in reversible addition
fragmentation chain transfer processes (RAFT), or alkoxyamines, asin
nitroxide mediated polymerization (NMP) [22].
In a typical ATRP, the dormant chain is terminated as an alkyl halide.
The dormant chain interacts with a transition metal in an oxidation/reduction
equilibrium (one electron oxidation of the transition metal) to generate the
polymer radical which can react with monomer and propagate. The propagat-
ing radical reacts with the oxidized transition metal to form a new dormant
chain terminated as an alkyl halide. Because the propagating radical exists
mainly in the dormant state, there is a relatively low concentration of the
active radical and therefore fewer opportunities for termination due to chain
coupling or disproportionation.
Perhaps the most common technique is the reversible addition fragmenta-
tion chain transfer (RAFT) polymerization [23]. A RAFT polymerization is
similar to a typical radical polymerization in that an initiator generates rad-
icals which then combine with monomer to form growing polymer chains.
A typical radical polymerization continues until termination occurs either
by radical combination or by disproportionation. The length of each chain
is statistical and there is typically a mixture of polymeric chains of a broad
molecular weight distribution. The distinction is that a RAFT polymerization
is carried out in the presence of a chain transfer agent, sometimes called a
RAFT agent. Trithiocarbonates are commonly used RAFT agents. A growing
polymer chain (Pol
.
) combines with the RAFT agent. This creates a dormant
species which does not undergo termination. It also generates another radical
which can react with any available monomer to create a second growing poly-
mer chain (Pol
2
.
). The second growing chain can combine with the dormant














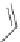








Search WWH ::

Custom Search