Biology Reference
In-Depth Information
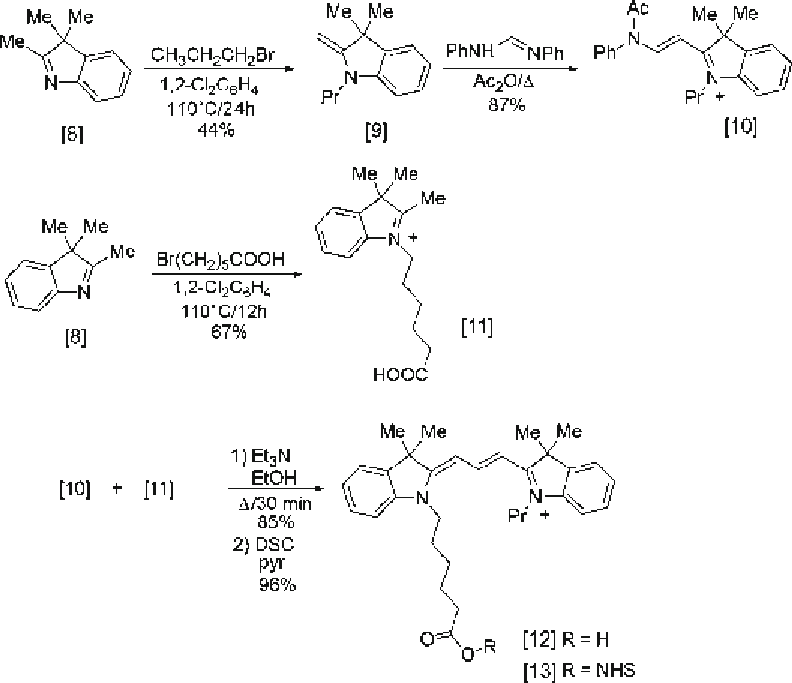
Fig. 2. Synthesis of the propyl Cy3 dye and its NHS ester.
1,119, 931, 767/cm.
1
H NMR (400 MHz, D
2
O): d 7.66 (1H,
m), 7.60 (1H, m), 7.45-7.50 (2H, m), 4.65 (2H, s), 4.30
(2H, t,
J
= 7.4 Hz), 1.86 (2H, m), 1.42 (6H, s), 0.87 (3H, t,
J
= 7.4 Hz).
13
C NMR (100 MHz, D
2
O): d 141.77, 140.92,
129.75, 128.99, 123.26, 115.15, 54.33, 49.20, 21.72, 20.85,
10.18.
B.
2-(2-Phenylacetamido-E-1-ethenyl)-3,3-dimethyl-1-propylin-
dolium salt
(10)
. A mixture of
N
,
N
¢-diphenylformamidine
(Aldrich, 0.35 g, 1.78 mmol) and 3,3-dimethyl-2-methylene-1-
propylindoline
(9)
(0.30 g, 1.48 mmol) in acetic anhydride
(10 mL) was refl uxed for 30 min. The solution was cooled to
room temperature, the solvent was removed under reduced
pressure, and the residue was purifi ed by fl ash chromatography
on silica gel (dichloromethane/hexane/methanol=5:1:1) to
give the salt
(10)
as a light yellow powder (0.45 g, 87%). IR
(neat): 2,965, 2,926, 1,680, 1,638, 1,603, 1,580, 1,553, 1,492,
Search WWH ::

Custom Search