Agriculture Reference
In-Depth Information
Souriau, 2003). These are generated by immunizing an animal with the antigen of interest.
Once a sufficient immune response is detected, the spleen, bone marrow from long bones
(femur and humerus), or primary lymphoid organs (such as lymph nodes) are removed from
the sacrificed animal and the antibody-producing B-cells are harvested. These cells can then
be fused to immortal myeloma cells (using an electrical current or by using polyethylene
glycol). The resulting hybrid cells (hybridomas), which secrete antibodies that are directed
toward the desired antigen, are then selected and cloned out to ensure monoclonality. The
advantage of this approach is that there is a constant supply of the antibody that is required
for analysis. However, there is a significant cost involved in the production and the screening
of these antibodies.
Recombinant antibodies, expressed in bacterial strains such as
Escherichia coli
,as
well as fungal and mammalian cells, are a very effective alternative. In 2003, 30% of
the antibodies used in clinical trials in the biopharmaceutical industry were recombinant
(Hudson and Souriau, 2003). These antibodies are employed in a phage-display format
(Bradbury and Marks, 2004).
The ability of molecular biology to increase the affinity of an antibody for an antigen has
permitted the use of a variety of high-affinity antibodies in biosensor-based platforms. The
amino acid sequence of a CDR region may be altered to enhance the binding characteristics
of an antibody by site-directed mutagenesis. Furthermore, antibody fragments (Fig. 20.6)
may be generated through the enzymatic digestion of an antibody by papain, which targets
V
H
V
L
scFv
V
H
V
L
C
H1
C
L
Fab
V
H
V
L
C
H1
C
L
C
H2
F(ab')
2
Fig. 20.6
Different antibody fragments that may be used for biosensors. The F(ab
)
2
is obtained through papain
digestion. The Fab and scFv are derived through recombinant techniques.






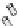

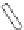
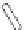























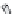


















Search WWH ::

Custom Search