Biomedical Engineering Reference
In-Depth Information
Al
2
O
3
, however, can also be grown with
plasma-assisted ALD. Here, the second precursor
is not water but oxygen plasma, produced
in situ
and which, due to the oxygen radicals produced,
shows enhanced reactivity toward TMA and
allows a lower processing temperature. The
quality of the coating may also be improved,
since the radical species tend to react more
vigorously with the ligands of the precursor or
the reaction byproducts and remove those from
the film. The plasma-assisted ALD, however,
requires more complex instrumentation and is
often not as easy to handle as thermal ALD. In
addition, the reacting species may sometimes
have a deleterious impact on the substrates,
particularly when those are polymeric. Also, due
to the shorter lifetime of the radical species,
coatings of deep pores and trenches may not be
as conformal as the corresponding thermal ALD
processes, since the radicals may quickly
recombine even before reaching the bottom of
such pores and trenches. Nevertheless, the
plasma-assisted ALD processes show good
promise for coatings at lower temperatures,
especially if metal coatings are required or the
chemical purity of the coating is of importance for
the anticipated application
[10]
.
From the chemical point of view, two general
cases may be differentiated. In the more com-
mon case, ALD will result in the growth of inor-
ganic materials, such as metal oxides, nitrides,
sulfides, or even metals
[9]
. This is simply
dependent on the selection of the precursors and
the thermal budget. In the currently less com-
mon case, organic molecules are used as precur-
sors. The move to reactive organic molecules
allows the layer-by-layer growth of polymers.
To differentiate these two growth processes,
the organic ALD is called
molecular layer deposi-
tion
(MLD). The first examples of MLD showed
the deposition of polyamides by alternating
injection of organic acid chlorides and amines
[11]
. The homo-bifunctionality of the molecules
used ensured that the growth was self-limiting.
More recently, the MLD was combined with
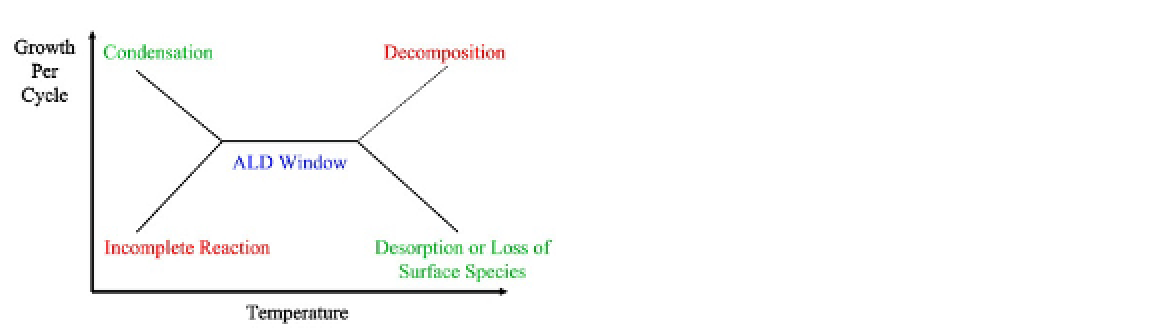
FIGURE 16.3
Schematic of the relation of the ALD
growth per cycle versus temperature. The scheme shows the
ALD window with possible scenarios for exceeding the tem-
perature limits at the lower and upper ends. Reprinted from
Ref.
5
. Copyright © 2010, with permission from the American
Chemical Society.
substrate due to the enhanced thermal budget.
Therefore, the ALD process is self-limiting and
reproducible only in a certain temperature range
for a particular pair of precursors. However,
within this range the growth is reproducible, and
the self-limiting nature is based on the interface
chemistry only.
16.1.3 Chemistry of ALD Processes
The chemistry involved in ALD is in most
cases based on simple chemical reactions such
as hydrolysis. In a few cases, the reactions are
redox reactions or condensation reactions. Tech-
nologically, most of the ALD processes can be
described as either thermal ALD, which is the
most common case as described thus far, or
the plasma-enhanced or plasma-assisted ALD,
where the second precursor is pushed toward
higher reactivity by generating a plasma
[5]
.
For thermal ALD of an Al
2
O
3
coating
[9]
, a
common first precursor is trimethylaluminum
(TMA), which is a highly volatile and pyrophoric
compound, and the second precursor is water.
The pair of precursors reacts to produce Al
2
O
3
and the growth can be easily controlled in a tem-
perature window between 100 °C and 300 °C.
Each cycle adds approximately 1 Å of Al
2
O
3
on
top of the substrate.