Biomedical Engineering Reference
In-Depth Information
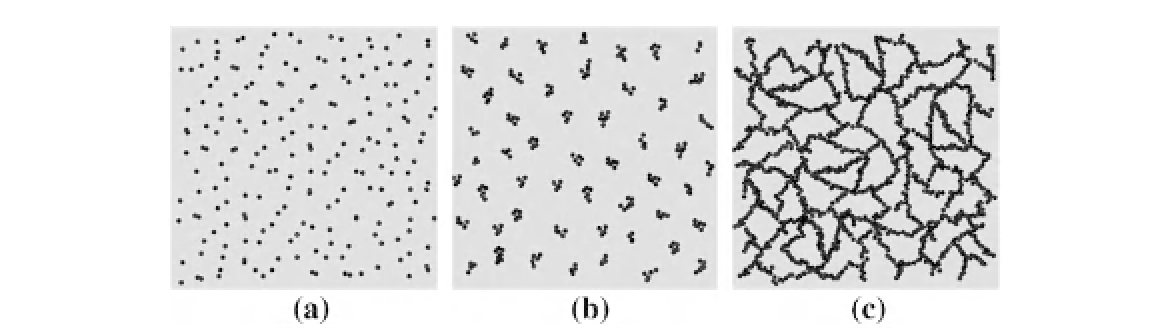
FIGURE 14.7
The three main stages of molecular sol-gel chemistry. (a) Solution stage: Partly hydrolyzed and condensed
molecular precursor species forming dimers and trimers (black dots). (b) Sol stage: Dimers and trimers agglomerate into
larger oligomers/nanoparticles. (c) Gel stage: Oligomers/nanoparticles further cross-link into an interconnected solid
network.
processing sol-gel compounds into desired
structures. To successfully transform the sol into
thin films, fibers, or monoliths or to infiltrate it
into template structures to create high-fidelity
ceramic replicas, gelation and formation of the
interconnected particle network need to be
slowed. This prevents premature solidification
and/or uncontrolled precipitation of the sol-gel
compound. In general, gelation kinetics depends
on similar parameters, as previously discussed
for the hydrolysis and condensation reactions.
Separating these processes is very difficult.
For templating applications, the most success-
ful approach is to dilute the sol either during or
immediately after the initial hydrolysis and con-
densation steps with low-boiling-point alcohols
(methanol, ethanol, isopropanol). Lowering the
colloidal particle concentration slows cross-link-
ing and allows template infiltration to be sepa-
rated from the gelation and solidification
processes. After infiltration of the template struc-
ture, alcohol evaporates and thereby slowly con-
centrates colloidal particles in the sol, inducing
their agglomeration and cross-linking into an
extended solid network, as shown in
Figure 14.7
.
A disadvantage of this method is that large
amounts of solvent are introduced along with
the sol-gel compound into the void space of
the template structure, resulting in a highly
porous and crack-prone network after solvent
evaporation. This interplay between gelation
kinetics and sol dilution depends strongly on
the sol-gel compound. Finding the appropriate
balance between these competing considera-
tions is one of the most important steps for
successful replication of synthetic and natural
structures.
Finally, it should be noted that for some sol-
gel compounds such as titania, the hydrolysis of
precursors (as well as condensation and gelation
reactions) are so fast that dilution alone is not
sufficient to prevent premature cross-linking
and precipitation. In these systems, it is impor-
tant to convert precursor molecules into inter-
mediary species that are stable under processing
conditions (coating, molding, casting, etc.) and
need other stimuli (such as heat and catalysts)
to undergo polymerization reactions. In the case
of titania, this can be achieved by converting
titanium alkoxides into stable oxy-chloro com-
plexes under strong acidic conditions or into
organic complexes by reaction with trifluoro-
acetic acid
[42]
. These entities are stable under
room-temperature conditions and can be pro-
cessed in an alcoholic solution. At elevated tem-
peratures, these complexes then decompose and
convert into amorphous or polycrystalline
titania.