Biomedical Engineering Reference
In-Depth Information
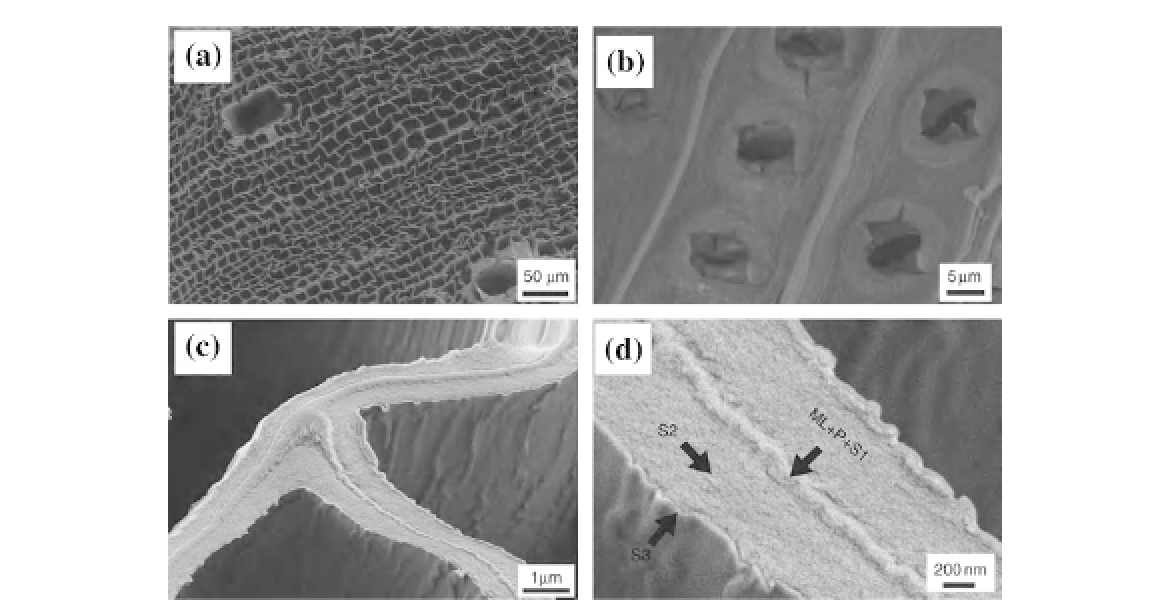
FIGURE 14.6
SEM images (a-d) of cerium/zirconium oxide replicas of spruce wood fabricated by nanoparticle sol
templating. Interfaces between different wood cell-wall layers are clearly visible in the ceramic replica samples. Adapted
from Ref.
33
. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.
infiltration, an increased solution viscosity can
significantly lower the degree of infiltration,
which, in turn, results in poor replica quality.
The optimum nanoparticle concentration
depends on the particle type and surface chem-
istry, but typical values are in the micro-to-
millimolar range
[10, 31]
.
After infiltration and evaporation of the
solvent, nanoparticles form an initially loosely
connected framework
via
interaction of surface
hydroxyl groups. This network can be further
densified and solidified by thermal treatment,
resulting in a stable nanoparticle-based struc-
ture held together by interparticle oxide bonds.
For example,
Figure 14.6
shows SEM images of
ceramic replicas of wood created by infiltration
of wood tissue with a sol containing cerium/
zirconium oxide nanoparticle sol and calcina-
tion (i.e. heating in air)
[31]
.
A different approach to creating inorganic
replicas is to infiltrate template structures with
molten or supersaturated salt solutions. For
example, heated supersaturated solutions of
salts, such as sodium chloride in water, are used
to infiltrate polymeric templates. After solvent
evaporation, solute precipitation, and thermal
removal of the template, an inverse replica of the
original structure composed of rock salt is
obtained
[35]
. In a similar method, sucrose was
infiltrated into diatom structures. The sucrose
was then carbonized, and after dissolution of
the diatom, a carbonaceous inverse replica was
obtained
[36]
.
Electrochemical deposition is an attractive
method to replicate open-framework template
structures into metals. Metal infiltration is
achieved with a traditional electrochemical cell
set-up in which the template-electrode is
immersed into an electrolyte solution containing
the metal salt of interest, together with a counter
electrode and reference electrode. Although this
technique is difficult to apply to biotemplates,
because it requires the template to be deposited
onto or formed on a conductive electrode, it has