Biomedical Engineering Reference
In-Depth Information
chitosan is a copolymer of glucosamine and
N
-acetyglucosamine units linked by 1-4 gluco-
sidic bonds and can be obtained by
N
-deacetyla-
tion of chitin.
Chitin
is the second most abundant
natural polymer on Earth. Chitosan is a polysac-
charide derived from chitin, part of the shell
structure of crustaceans and shellfish. The chi-
tosan is produced commercially by deacetylation
of chitin. Chitosan is also a cationic polyeletro-
lyte. The degree of deacetylation can be deter-
mined by NMR spectroscopy and can vary from
60% to 100%.
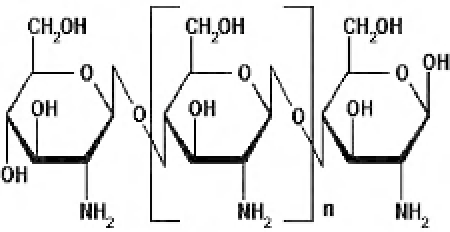
The structure of chitosan is similar to that of
cellulose, with the presence of amino groups
being the major difference (
Figure 6.1
). The fact
that chitosan may be made electroactive with
sensing and actuation capability is evidenced by
the work of Cai and Kim
[40]
on electoactive
papers based on cellulose, as well as the work
of Mac and Sun
[31]
on chitosan gels. Chitosan
is structurally related to cellulose, which con-
sists of long chains of glucose molecules linked
to each other.
FIGURE 6.1
General structure of chitosan polyelectrolyte.
Chitosan comprises copolymers of
N
-acetyl-
glucosamine and glucosamine and is a linear
natural polysaccharide. Chitosan is prepared
from chitin, which is closely related to both
chitosan, a more water soluble derivative of
chitin, and to cellulose, since it is a long
unbranched chain of glucose derivatives, shown
in
Figure 6.2
.
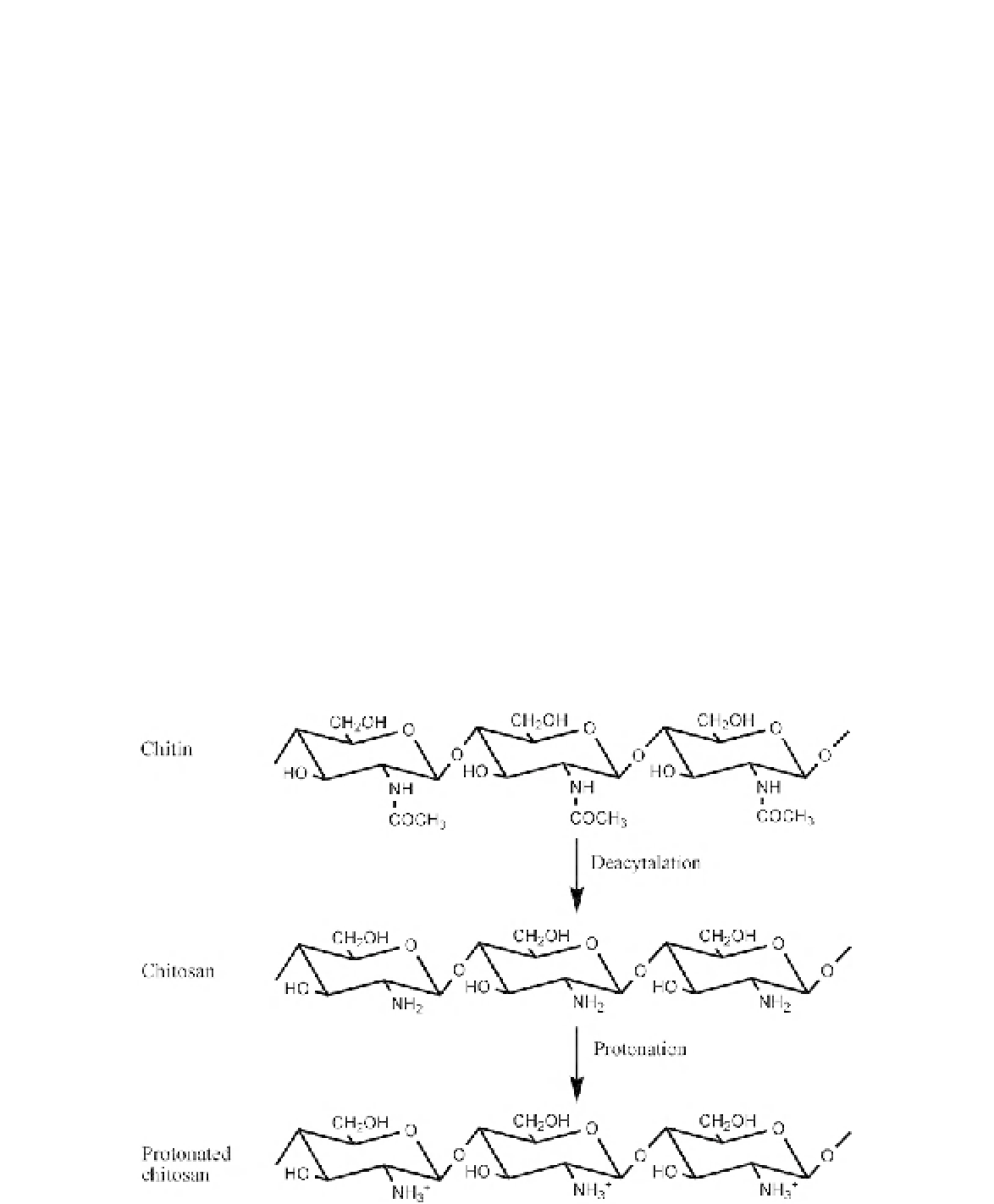
Note that protonated chitosan is cationic
and is positively charged. These properties
make chitosan ideal for use as a bio-adhesive,
as it bonds to negatively charged surfaces such
as mucosal membranes. Several studies have
FIGURE 6.2
Manufacturing protonated chitosan from chitin by deacetylation in NaOH
[30].