Geoscience Reference
In-Depth Information
Real Gas Pseudopressures
The real gas pseudopressure was defined by Al-Hussainy et al. to allow
solutions to the diffusivity equation originally developed for slightly
compressible liquids to be applied to flow in gas reservoirs.
39
This fluid
property is used primarily in pressure transient analysis.
(2.24)
The lower limit on the integral can be taken to be 0.0 if the Lee,
Gonzalez, Eakin equations are used to estimate gas viscosity. The units
of pressure are pounds per square inch absolute (psia), and gas viscosity
units are centipoises, so the units of real gas pseudopressure are pounds
per square inch squared per centipoises (psi
2
/cp). This equation is not a
correlation; it is simply a part of the larger gas diffusivity equation. The
integral can be evaluated numerically using values of gas viscosities and
gas z-factors from the correlation equations and procedures discussed
previously. The resulting values of
m(p)
are as accurate as the values of
gas viscosities and gas z-factors used in the calculations.
Gas Properties at High Pressures and High
Temperatures, HPHT
HPHT reservoirs are generally considered to have pressures from
10,000 psia to 30,000 psia and temperatures above 300ºF. Currently no
published data of gas z-factors or gas viscosities for naturally occurring
petroleum gases are available for use in preparing correlations for use at
HPHT conditions.
Gas densities (z-factors) at HPHT
The hydrocarbon portion of gases that are found in HPHT reservoirs
is essentially pure methane, although some level of nonhydrocarbon
components is usually present.
The National Institute of Standards and Technology, NIST, has
prepared tables of density and viscosity values for pure methane.
40
Gas
densities calculated with the Piper et al. and DAK equations [equations
(2.3)-(2.8)] were tested against the NIST methane density values at


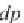







































































Search WWH ::

Custom Search