Geoscience Reference
In-Depth Information
Step 15.
Next, calculate the derivative of the molar volume of methane
dissolved in brine with respect to pressure from equation (4.31).
,
(4.31)
where
m
Na
is the concentration of sodium ions in g-mol/kg H
2
O
(numerically equal to
m
NaCl
for a brine containing only sodium chloride).
Step 16.
Calculate the compressibility of undersaturated brine,
c
wu
, in
MPa
-1
, from equation (4.32).
(4.32)
The second case of interest for compressibility of brine with dissolved
methane is that of a two-phase system with brine in equilibrium with
methane gas. In this case, the derivative with respect to pressure in
equation (4.26) is taken assuming that the brine and gas phases remain in
equilibrium. Thus, the change in methane concentration with pressure
must be accounted for in taking the derivative in equation (4.26).
Step 17.
First, calculate the derivative of the methane solubility with
respect to pressure, as shown in equation (4.33).
(4.33)
,
where
p
σ
is the vapor pressure of pure water calculated in Step 8, and
A
(
T
) and
B
(
T
) are temperature-dependent coefficients calculated in Step
9.

































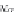




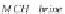




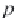

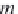

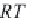
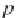


























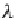
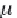




















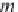
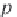

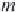
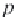











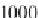
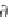

















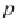
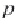
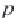


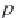
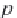
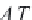
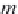
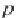
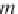

























































































Search WWH ::

Custom Search