Geoscience Reference
In-Depth Information
Density of brine containing dissolved methane
The most straightforward way to calculate the density of brine
containing dissolved methane is to calculate the mass and volume of
a sample of brine containing 1,000 grams pure water, then calculate
the density of the brine from the mass and volume. The following steps
give the correct density for brines either saturated or undersaturated
with methane.
Step 11.
First, calculate the derivatives with respect to pressure for the
terms ,
λ
CH
4
,Na
, and
ζ
CH
4
,NaCl
, using equations (4.19)-(4.21), along
with the appropriate coefficients from table 4-11. The coefficients for
the term
are from Duan-Mao, converted to pressures in MPa.
25
Again, note that temperature in equation (4.19) is Kelvin.
(4.19)
(4.20)
(4.21)
Next, calculate the partial molar volume of methane in brine, in
cm
3
/g-mol, using equation (4.22), from Duan.
26
Here,
R
is the universal
gas constant,
R
= 8.314467 MPa⋅cm
3
/ g-mol⋅K, and
T
is the temperature
in Kelvin. The concentration of sodium ions is
m
Na
, in g-mole/kg H
2
O,
and is numerically equal to the salinity
m
NaCl
, since a sodium chloride
brine is assumed.
(4.22)
























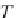

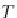

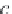











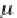
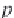











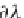




































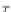


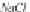







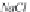
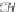





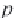



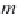






















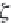
















































































Search WWH ::

Custom Search